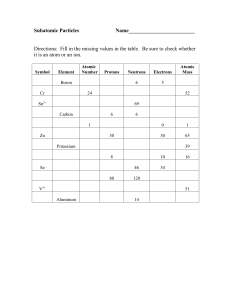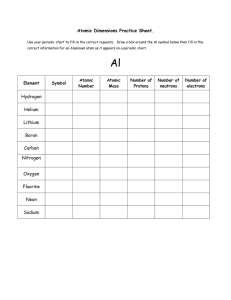
Quiz review
... Periodic Trends Quiz review The “Father of the Periodic Table” (a Russian) – spell his name correctly! Horizontal rows of the periodic table are called this. Vertical columns of the periodic table are called ‘groups’ or this. Which element in period 3 has 6 valence electrons? Which element in period ...
... Periodic Trends Quiz review The “Father of the Periodic Table” (a Russian) – spell his name correctly! Horizontal rows of the periodic table are called this. Vertical columns of the periodic table are called ‘groups’ or this. Which element in period 3 has 6 valence electrons? Which element in period ...
Atomic Structure and Periodic Table Review Guide
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
Chemical reactions revision
... Element are arranged in the table in order of their atomic number Elements in different groups (columns) have different properties. Elements are often split into the groups metals and non-metals. Metals are strong, sonorous (ring), malleable (can be bent into shape) and are good conductors of heat a ...
... Element are arranged in the table in order of their atomic number Elements in different groups (columns) have different properties. Elements are often split into the groups metals and non-metals. Metals are strong, sonorous (ring), malleable (can be bent into shape) and are good conductors of heat a ...
Agenda/To Do - Perry Local Schools
... chemical properties between the elements. 2. Arranged the elements into rows in order of increasing mass so that elements of similar properties were in the same column. *created the first periodic table ...
... chemical properties between the elements. 2. Arranged the elements into rows in order of increasing mass so that elements of similar properties were in the same column. *created the first periodic table ...
Atom through Periodic Table Study Guide
... 17. Which family has elements that are highly reactive non-metals and has 7 valence electrons? 18. The noble gases are found in which column of the periodic table? 19. The noble gases are non-reactive- they won’t bond unless forced to. WHY won’t they bond? 20. The elements on the left side of the pe ...
... 17. Which family has elements that are highly reactive non-metals and has 7 valence electrons? 18. The noble gases are found in which column of the periodic table? 19. The noble gases are non-reactive- they won’t bond unless forced to. WHY won’t they bond? 20. The elements on the left side of the pe ...
Homework Geochem Test Review
... 15. What do we call the bond that forms when one atom give electrons to another atom? ...
... 15. What do we call the bond that forms when one atom give electrons to another atom? ...
Chapter 6 Notes
... NIB - Groups of elements and their Properties – Students should refer to Appendix A!!! Properties of families Group 1 - Alkali Metals - “alkali” comes from Arabic - means “ashes” - early chemists separated sodium and potassium compounds from ashes - the hydroxides of these compounds are strongly ba ...
... NIB - Groups of elements and their Properties – Students should refer to Appendix A!!! Properties of families Group 1 - Alkali Metals - “alkali” comes from Arabic - means “ashes” - early chemists separated sodium and potassium compounds from ashes - the hydroxides of these compounds are strongly ba ...
Ch. 5 Outline Notes
... a. Discovered by _____________________ gold foil experiment in 1911 b. Fired stream of positive particles at gold foil, most passed right through (atom mostly _____________ space) while a few bounced off (very _______________ positive nucleus at center) B. Development of Modern Atomic Theory 1. ____ ...
... a. Discovered by _____________________ gold foil experiment in 1911 b. Fired stream of positive particles at gold foil, most passed right through (atom mostly _____________ space) while a few bounced off (very _______________ positive nucleus at center) B. Development of Modern Atomic Theory 1. ____ ...
semester 1 study guide 2015 - slater science
... number of valence electrons, and the same oxidation number o Understand that reactivity increases down in a group of metals and decrease down in a group of nonmetals o Identify main group elements as A groups or as groups 1, 2, 13-18 o Identify alkali metals, alkaline earth metals, halogens, and nob ...
... number of valence electrons, and the same oxidation number o Understand that reactivity increases down in a group of metals and decrease down in a group of nonmetals o Identify main group elements as A groups or as groups 1, 2, 13-18 o Identify alkali metals, alkaline earth metals, halogens, and nob ...
Chemistry 1 – Tollett Chapter 5 – Atomic Structure & The Periodic
... Table was first put together by Demitri Mendeleev. ...
... Table was first put together by Demitri Mendeleev. ...
Chemistry Semester One Exam Review Name:
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
Chapter 4 Study Guide Physical Science 1. The word atom comes
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
PS-CC-2test - Edquest Science
... 12. As you move across the periodic table the properties of the elements change. The most reactive metals include … A. sodium and lithium B. iron and copper C. aluminum and carbon D. lead and zinc 13. The periodic table is organized by the patterns of the properties of the elements. The rows in the ...
... 12. As you move across the periodic table the properties of the elements change. The most reactive metals include … A. sodium and lithium B. iron and copper C. aluminum and carbon D. lead and zinc 13. The periodic table is organized by the patterns of the properties of the elements. The rows in the ...
Unit 1: Atomic Structure AP Chemistry
... Proportions Nearly discovered the Law of multiple proportions, but his data used percentages instead of weights. ...
... Proportions Nearly discovered the Law of multiple proportions, but his data used percentages instead of weights. ...
Periodic table
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number (number of protons in the nucleus), electron configurations, and recurring chemical properties. The table also shows four rectangular blocks: s-, p- d- and f-block. In general, within one row (period) the elements are metals on the lefthand side, and non-metals on the righthand side.The rows of the table are called periods; the columns are called groups. Six groups (columns) have names as well as numbers: for example, group 17 elements are the halogens; and group 18, the noble gases. The periodic table can be used to derive relationships between the properties of the elements, and predict the properties of new elements yet to be discovered or synthesized. The periodic table provides a useful framework for analyzing chemical behavior, and is widely used in chemistry and other sciences.Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. He developed his table to illustrate periodic trends in the properties of the then-known elements. Mendeleev also predicted some properties of then-unknown elements that would be expected to fill gaps in this table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev's periodic table has since been expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behavior.All elements from atomic numbers 1 (hydrogen) to 118 (ununoctium) have been discovered or reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 94 elements exist naturally, although some are found only in trace amounts and were synthesized in laboratories before being found in nature. Elements with atomic numbers from 95 to 118 have only been synthesized in laboratories. It has been shown that einsteinium and fermium once occurred in nature but currently do not. Synthesis of elements having higher atomic numbers is being pursued. Numerous synthetic radionuclides of naturally occurring elements have also been produced in laboratories.