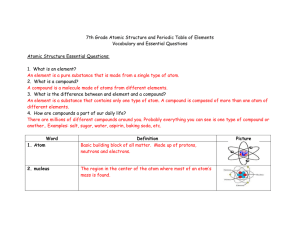
7th Grade Atomic Structure and Periodic Table of Elements
... A compound is a molecule made of atoms from different elements. 3. What is the difference between and element and a compound? An element is a substance that contains only one type of atom. A compound is composed of more than one atom of different elements. 4. How are compounds a part of our daily li ...
... A compound is a molecule made of atoms from different elements. 3. What is the difference between and element and a compound? An element is a substance that contains only one type of atom. A compound is composed of more than one atom of different elements. 4. How are compounds a part of our daily li ...
and View
... c. Symbol—abbreviation of the name. (Greek, Latin, or Location) d. Atomic mass—mass of protons with neutrons. C. IVPAC—organization that chooses permanent name for element. ...
... c. Symbol—abbreviation of the name. (Greek, Latin, or Location) d. Atomic mass—mass of protons with neutrons. C. IVPAC—organization that chooses permanent name for element. ...
The Periodic Table
... nucleons in an isotope and atomic mass is the measure of the average mass of an atom including the relative abundance of its element’s isotopes. ...
... nucleons in an isotope and atomic mass is the measure of the average mass of an atom including the relative abundance of its element’s isotopes. ...
3-ELEMENTS AND THE ATOMIC MODEL. C4.8A Identify the
... Identify properties of common families of elements. Identify properties of common periods on the periodic table. Explain the history and organization of the periodic table. C4.8e Write the complete electron configuration of elements in the first three rows of the periodic table. C4.8g Predict oxidat ...
... Identify properties of common families of elements. Identify properties of common periods on the periodic table. Explain the history and organization of the periodic table. C4.8e Write the complete electron configuration of elements in the first three rows of the periodic table. C4.8g Predict oxidat ...
Test 2 Review Test 2 Review (15-16)_2
... (III) Dalton’s Atomic Theory suggests each of the following points. Explain whether any of these points are false and if they are false explain why we know them to be false now. (see p. 103 & 112) (i) All elements are composed of atoms which cannot be broken into smaller parts. (ii) All atoms of the ...
... (III) Dalton’s Atomic Theory suggests each of the following points. Explain whether any of these points are false and if they are false explain why we know them to be false now. (see p. 103 & 112) (i) All elements are composed of atoms which cannot be broken into smaller parts. (ii) All atoms of the ...
No Slide Title
... Periodic Table 30 • Which group of elements share characteristics of both metals and nonmetals? • What are metalloids or semi-metals? ...
... Periodic Table 30 • Which group of elements share characteristics of both metals and nonmetals? • What are metalloids or semi-metals? ...
atomic number
... Dalton’s Atomic Theory – Atoms of different elements can be distinguished by their different masses – Compounds are combinations of atoms of different elements and possess properties different from those of their component elements – In chemical reactions, atoms are neither created nor destroyed bu ...
... Dalton’s Atomic Theory – Atoms of different elements can be distinguished by their different masses – Compounds are combinations of atoms of different elements and possess properties different from those of their component elements – In chemical reactions, atoms are neither created nor destroyed bu ...
Metals, Nonmetals, and Metalloids (Vocabulary)
... Metals, Nonmetals, and Metalloids (Vocabulary) ...
... Metals, Nonmetals, and Metalloids (Vocabulary) ...
Chemistry Notes
... stating that when two elements can combine to form more than one compound the amounts of one of them that combines with a fixed amount of the other will exhibit a simple multiple relation ...
... stating that when two elements can combine to form more than one compound the amounts of one of them that combines with a fixed amount of the other will exhibit a simple multiple relation ...
Vocabulary for Periodic Table
... 7) Isotope: atoms of the same element that have a different number of neutrons. 8) Ion: when an atom loses or gains electrons. 9) Atomic mass: the average mass of the atoms in an element. 10) Periodic Table: a table of elements, arranged by atomic number, that shows the patterns in their properties. ...
... 7) Isotope: atoms of the same element that have a different number of neutrons. 8) Ion: when an atom loses or gains electrons. 9) Atomic mass: the average mass of the atoms in an element. 10) Periodic Table: a table of elements, arranged by atomic number, that shows the patterns in their properties. ...
Metals, Nonmetals, and Metalloids (Vocabulary)
... are arranged by properties and are represented by one or two letter chemical symbols. ...
... are arranged by properties and are represented by one or two letter chemical symbols. ...
Learning Standards vocab chemical basis and molecules of life 09
... Given the number of protons, identify the element using a Periodic Table. Explain the arrangement of the elements on the Periodic Table, including the significant relationships among elements in a given column or row. Explain how ions and ionic bonds are formed (e.g., sodium atoms lose an elec ...
... Given the number of protons, identify the element using a Periodic Table. Explain the arrangement of the elements on the Periodic Table, including the significant relationships among elements in a given column or row. Explain how ions and ionic bonds are formed (e.g., sodium atoms lose an elec ...
Chemistry Study Guide
... react with other forms of matter. For example, some substances are flammable. If they are heated with oxygen, they will react and burst into flames. The ability of a substance to combine with oxygen is an example of chemical property. ...
... react with other forms of matter. For example, some substances are flammable. If they are heated with oxygen, they will react and burst into flames. The ability of a substance to combine with oxygen is an example of chemical property. ...
Chemistry Study Guide
... react with other forms of matter. For example, some substances are flammable. If they are heated with oxygen, they will react and burst into flames. The ability of a substance to combine with oxygen is an example of chemical property. ...
... react with other forms of matter. For example, some substances are flammable. If they are heated with oxygen, they will react and burst into flames. The ability of a substance to combine with oxygen is an example of chemical property. ...
Periodic Table Vocabulary Periodic Table – a chart that organizes
... chemical reaction, matter cannot be created or destroyed but can be changed into a different form. Period law- The chemical properties of elements tends to repeat over specific atomic number intervals Law of definite proportions – elements within a compound have specific mass ratios Isotopes Atoms o ...
... chemical reaction, matter cannot be created or destroyed but can be changed into a different form. Period law- The chemical properties of elements tends to repeat over specific atomic number intervals Law of definite proportions – elements within a compound have specific mass ratios Isotopes Atoms o ...
Groups of the Periodic Table
... • Have some properties of metals AND some of non-metals • Some are very good conductors, others are very poor – used as semiconductors in circuits and lasers ...
... • Have some properties of metals AND some of non-metals • Some are very good conductors, others are very poor – used as semiconductors in circuits and lasers ...
– Units 5-7 Review Honors Chemistry Unit 5
... The element copper is found to contain the naturally occurring isotopes 29Cu63 and 29Cu65. The relative abundances are 69.1% and 30.9% respectively. Calculate the average atomic mass of copper. Ninety-two percent of the atoms of an element have a mass of 28.0 amu, 5.0% of the atoms have a mass of 29 ...
... The element copper is found to contain the naturally occurring isotopes 29Cu63 and 29Cu65. The relative abundances are 69.1% and 30.9% respectively. Calculate the average atomic mass of copper. Ninety-two percent of the atoms of an element have a mass of 28.0 amu, 5.0% of the atoms have a mass of 29 ...
The Chemical Basis of Life Chapter 4
... How is Matter Organized? •Divided into pure substances or mixtures based on composition. –Pure substance •Uniform composition with same properties throughout •Can be classified as either an element or a compound ...
... How is Matter Organized? •Divided into pure substances or mixtures based on composition. –Pure substance •Uniform composition with same properties throughout •Can be classified as either an element or a compound ...
Periodic table
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number (number of protons in the nucleus), electron configurations, and recurring chemical properties. The table also shows four rectangular blocks: s-, p- d- and f-block. In general, within one row (period) the elements are metals on the lefthand side, and non-metals on the righthand side.The rows of the table are called periods; the columns are called groups. Six groups (columns) have names as well as numbers: for example, group 17 elements are the halogens; and group 18, the noble gases. The periodic table can be used to derive relationships between the properties of the elements, and predict the properties of new elements yet to be discovered or synthesized. The periodic table provides a useful framework for analyzing chemical behavior, and is widely used in chemistry and other sciences.Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. He developed his table to illustrate periodic trends in the properties of the then-known elements. Mendeleev also predicted some properties of then-unknown elements that would be expected to fill gaps in this table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev's periodic table has since been expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behavior.All elements from atomic numbers 1 (hydrogen) to 118 (ununoctium) have been discovered or reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 94 elements exist naturally, although some are found only in trace amounts and were synthesized in laboratories before being found in nature. Elements with atomic numbers from 95 to 118 have only been synthesized in laboratories. It has been shown that einsteinium and fermium once occurred in nature but currently do not. Synthesis of elements having higher atomic numbers is being pursued. Numerous synthetic radionuclides of naturally occurring elements have also been produced in laboratories.