AP Biology - John D. O`Bryant School of Math & Science
... and the product of the particular citric acid cycle step involved.) Propose a possible way to solve this deficiency. ...
... and the product of the particular citric acid cycle step involved.) Propose a possible way to solve this deficiency. ...
Cellular Respiration
... cells. Their number within the cell ranges from a few hundred to, in very active cells, thousands. Their main function is the conversion of the potential energy of food molecules into ATP. ...
... cells. Their number within the cell ranges from a few hundred to, in very active cells, thousands. Their main function is the conversion of the potential energy of food molecules into ATP. ...
chapter9_powerpoint
... • Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space • H+ then moves back across the membrane, passing through the protein/enzyme, ATP synthase • ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ...
... • Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space • H+ then moves back across the membrane, passing through the protein/enzyme, ATP synthase • ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ...
fatty acid metabolism
... FA synthesis – cytoplasmic – Citrate Shuttle • moves AcCoA to cytoplasm • produces 50% NADPH via malic enzyme ...
... FA synthesis – cytoplasmic – Citrate Shuttle • moves AcCoA to cytoplasm • produces 50% NADPH via malic enzyme ...
Metabolism Aerobic Respiration Other Ways of Generating ATP
... Glycolysis used to generate ATP NAD+ reduced to NADH Must oxidize NADH back to NAD+ Reduce pyruvate into lactate Aquatic invertebrates • more complex pathways • Involve Krebs cycle reactions and truncated electron transport activity ...
... Glycolysis used to generate ATP NAD+ reduced to NADH Must oxidize NADH back to NAD+ Reduce pyruvate into lactate Aquatic invertebrates • more complex pathways • Involve Krebs cycle reactions and truncated electron transport activity ...
Respiration
... mitochondrial membrane – Lower actual P/O ratios: ~2.5 for NADH and 1.5 for FADH2 ...
... mitochondrial membrane – Lower actual P/O ratios: ~2.5 for NADH and 1.5 for FADH2 ...
METABOLISM CATABOLISM AND ANABOLISM ATP MOLECULE
... Glucose catabolism – a series of small steps, each controlled by a separate enzyme, in which energy is released in small manageable amounts, and as much as possible, is transferred to ATP and the rest is released as heat Three major pathways of glucose catabolism ...
... Glucose catabolism – a series of small steps, each controlled by a separate enzyme, in which energy is released in small manageable amounts, and as much as possible, is transferred to ATP and the rest is released as heat Three major pathways of glucose catabolism ...
Dear Notetaker:
... a. The mitochondria when citrate builds up i. If Krebs cycle has enough energy, it slows down, citrate builds up, and acetyl CoA can leave then. Regulatory step that is important..need to understand!! 7. How many moles of ATP are produced when one mole of glucose is completely oxidized to carbon dio ...
... a. The mitochondria when citrate builds up i. If Krebs cycle has enough energy, it slows down, citrate builds up, and acetyl CoA can leave then. Regulatory step that is important..need to understand!! 7. How many moles of ATP are produced when one mole of glucose is completely oxidized to carbon dio ...
Pathology Ketone bodies are created at moderate
... can be converted to glucose when needed. There are also small stores of glycogen in muscle tissue. When necessary protein can be stripped from muscle to convert to glucose in times of extreme need. Likewise fatty acids can be converted to ketones by breaking down fat stored in adipose tissue. It may ...
... can be converted to glucose when needed. There are also small stores of glycogen in muscle tissue. When necessary protein can be stripped from muscle to convert to glucose in times of extreme need. Likewise fatty acids can be converted to ketones by breaking down fat stored in adipose tissue. It may ...
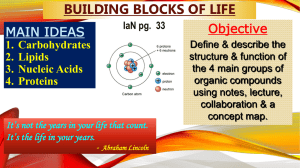
Macromolecules Vocabulary and Concepts
... o Ring form of glucose comes in two forms: alpha and beta glucose o Starch: polymer of alpha glucose, energy storage in plants, digested by animals o Glycogen: polymer of alpha glucose, energy storage in animals o Cellulose: polymer of beta glucose, structural component of plants, not digested by an ...
... o Ring form of glucose comes in two forms: alpha and beta glucose o Starch: polymer of alpha glucose, energy storage in plants, digested by animals o Glycogen: polymer of alpha glucose, energy storage in animals o Cellulose: polymer of beta glucose, structural component of plants, not digested by an ...
PART 1: TRUE OR FALSE (1 point each)
... which a molecule of water is generated. 2. In living organisms, the majority of proteins found exist in only one isomeric form. 3. Within a single protein, both alpha helices and beta sheets can be present. 4. Noncovalent bonds are the main determinant of protein tertiary structure. 5. According to ...
... which a molecule of water is generated. 2. In living organisms, the majority of proteins found exist in only one isomeric form. 3. Within a single protein, both alpha helices and beta sheets can be present. 4. Noncovalent bonds are the main determinant of protein tertiary structure. 5. According to ...
Glucose
... a) Ouabin (cardiac glycoside): Inhibits adenosine triphosphatase (ATPase) necessary for hydrolysis of ATP that produces energy of sodium pump. b) Phlorhizin; Inhibits the binding of sodium in the carrier protein. B. Passive transport (facilitated diffusion): Sugars pass with concentration gradient i ...
... a) Ouabin (cardiac glycoside): Inhibits adenosine triphosphatase (ATPase) necessary for hydrolysis of ATP that produces energy of sodium pump. b) Phlorhizin; Inhibits the binding of sodium in the carrier protein. B. Passive transport (facilitated diffusion): Sugars pass with concentration gradient i ...
Metabolism Part II: The tricarboxylic acid (TCA), citric acid, or Krebs
... metabolize intermediates present in excess is through anaplerotic (literally: filling up) reactions which provide shortcuts across the cycle. The most important example of anaplerosis is a reversible reaction in which pyruvate is converted to oxaloacetate by the enzyme pyruvate carboxylase. ...
... metabolize intermediates present in excess is through anaplerotic (literally: filling up) reactions which provide shortcuts across the cycle. The most important example of anaplerosis is a reversible reaction in which pyruvate is converted to oxaloacetate by the enzyme pyruvate carboxylase. ...
(pt=2) Define photosynthesis
... What are 2 primary structures seen in A? a) _________________ b) ___________________ If the codon in A is GCA, what is the anit-codon? _____________ What is the amino acid?_________ What is the primary process seen in B? ______________________________________________________________________________ ...
... What are 2 primary structures seen in A? a) _________________ b) ___________________ If the codon in A is GCA, what is the anit-codon? _____________ What is the amino acid?_________ What is the primary process seen in B? ______________________________________________________________________________ ...
Biology Review Test
... 8. What is the equation that summarizes cellular respiration? a. C6H12O6 + 6 O2 6 CO2 + 6 H2O + Energy b. 6 CO2 + 6 H2O + Energy C6H12O6 + 6 O2 c. ADP + Pi + Energy ATP d. C6H12O6 Lactic Acid + 2 ATP ...
... 8. What is the equation that summarizes cellular respiration? a. C6H12O6 + 6 O2 6 CO2 + 6 H2O + Energy b. 6 CO2 + 6 H2O + Energy C6H12O6 + 6 O2 c. ADP + Pi + Energy ATP d. C6H12O6 Lactic Acid + 2 ATP ...
Document
... three increasing substrate concentrations. You find that the slope of the product versus time curve increases as concentration of substrate increases. What can you conclude about the conditions in the reaction mixture? Explain. Activity of phosphofructokinase, which catalyzes step 3 of glycolysis, i ...
... three increasing substrate concentrations. You find that the slope of the product versus time curve increases as concentration of substrate increases. What can you conclude about the conditions in the reaction mixture? Explain. Activity of phosphofructokinase, which catalyzes step 3 of glycolysis, i ...
2421_Ch5.ppt
... Coupling of these reactions is made possible through ATP So… what does he mean by coupling?” energy retrieved from catabolism is stored in ATP and later released to drive anabolic reactions ...
... Coupling of these reactions is made possible through ATP So… what does he mean by coupling?” energy retrieved from catabolism is stored in ATP and later released to drive anabolic reactions ...
Monday Oct
... • Glycolytic: at peak activity rely on glycolysis – few mitochondria, many glycolytic enzymes, large store of glycogen, fewer capillaries, little myoglobin (white) Metabolic profiles CAN BE modified by athletic training! ...
... • Glycolytic: at peak activity rely on glycolysis – few mitochondria, many glycolytic enzymes, large store of glycogen, fewer capillaries, little myoglobin (white) Metabolic profiles CAN BE modified by athletic training! ...
2-3 Notes B
... It’s not the years in your life that count. It’s the life in your years. - Abraham Lincoln ...
... It’s not the years in your life that count. It’s the life in your years. - Abraham Lincoln ...
You Light Up My Life - Hawaii Community College
... Final electron acceptor is compound from environment (such as nitrate), not oxygen ATP yield is low ...
... Final electron acceptor is compound from environment (such as nitrate), not oxygen ATP yield is low ...
Chapter 14 Glycolysis, Gluconeogenesis, and the Pentose
... formation. In extracts to which glucose is added, fermentation proceeds until ADP and Pi (present in the extracts) are exhausted. (a) Phosphate is required in the glyceraldehyde 3-phosphate dehydrogenase reaction, and glycolysis will stop at this step when Pi is exhausted. Because glucose remains, i ...
... formation. In extracts to which glucose is added, fermentation proceeds until ADP and Pi (present in the extracts) are exhausted. (a) Phosphate is required in the glyceraldehyde 3-phosphate dehydrogenase reaction, and glycolysis will stop at this step when Pi is exhausted. Because glucose remains, i ...
functional group
... Chitin is a N containing polysaccharide which is found in the exoskeleton of insects and cell wall of fungi. They are not soluble or slightly soluble. ...
... Chitin is a N containing polysaccharide which is found in the exoskeleton of insects and cell wall of fungi. They are not soluble or slightly soluble. ...
2 ATP - Loyola Blakefield
... protons from the Kreb’s cycle move to this chain-like a series of steps (staircase). As electrons drop down stairs, energy released to form a total of 32 ATP Oxygen waits at bottom of staircase, picks up electrons and protons and in doing so becomes ...
... protons from the Kreb’s cycle move to this chain-like a series of steps (staircase). As electrons drop down stairs, energy released to form a total of 32 ATP Oxygen waits at bottom of staircase, picks up electrons and protons and in doing so becomes ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑