Exam 1
... Know and be able draw the arrow pushing mechanism for these reactions: Electrophilic Aromatic Substitution (in making Azo dyes,), Nucleophilic acyl substitutions Acid catalyzed ester & amide hydrolysis, Fisher esterification, trans esterification Hydroxide promoted ester hydrolysis Nucleophilic addi ...
... Know and be able draw the arrow pushing mechanism for these reactions: Electrophilic Aromatic Substitution (in making Azo dyes,), Nucleophilic acyl substitutions Acid catalyzed ester & amide hydrolysis, Fisher esterification, trans esterification Hydroxide promoted ester hydrolysis Nucleophilic addi ...
C1_5_products_from_oils_crossword
... 9. The reaction in which the enzymes in yeast turn glucose into ethanol and carbon dioxide. 10. An alkene with the formula C3H6. 11. Polymers that change in response to changes in their environment. 12. A hydrocarbon whose molecules contain at least one carbon-carbon double bond. Down 1. Something t ...
... 9. The reaction in which the enzymes in yeast turn glucose into ethanol and carbon dioxide. 10. An alkene with the formula C3H6. 11. Polymers that change in response to changes in their environment. 12. A hydrocarbon whose molecules contain at least one carbon-carbon double bond. Down 1. Something t ...
Chap Thirteen: Alcohols
... ii. reaction with Ketones and Aldehydes iii. reaction with acid chlorides or esters (double addition) iv. reaction with epoxides (Anti stereoselective; SN2-like regioselectivity) v. Side reactions with acidic compounds d. Via reduction of carbonyls or epoxides with Hydride Reducing reagents i. Reduc ...
... ii. reaction with Ketones and Aldehydes iii. reaction with acid chlorides or esters (double addition) iv. reaction with epoxides (Anti stereoselective; SN2-like regioselectivity) v. Side reactions with acidic compounds d. Via reduction of carbonyls or epoxides with Hydride Reducing reagents i. Reduc ...
... 9. What is trans esterification. 10. Arrange the following in terms of increasing acid strength and give reasons. Propionic acid , 2chloropropionic acid , 2 fluoropropionic acid. PART - B Answer any EIGHT questions (8 x 5 = 40) 11. Give a mechanism for the reaction of tert.butyl bromide with aqueous ...
International Arab Baccalaureate
... acid and the appropriate amine, whereas in hydrolysis a molecule is cleaved into two parts by the addition of a molecule of water. Question 2: Amides can be hydrolyzed under acidic or basic conditions. Describe the reaction of hydrolysis and give the obtained compounds in acidic medium. ...
... acid and the appropriate amine, whereas in hydrolysis a molecule is cleaved into two parts by the addition of a molecule of water. Question 2: Amides can be hydrolyzed under acidic or basic conditions. Describe the reaction of hydrolysis and give the obtained compounds in acidic medium. ...
What is an addition reaction
... Halogenatated alkane Halogen Halogenation Double Halogenated alkane Condensation (also called Elimination) In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must ha ...
... Halogenatated alkane Halogen Halogenation Double Halogenated alkane Condensation (also called Elimination) In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must ha ...
Chapter 20 Amines-part 2
... (H 3PO2) to replace the diazonium group with a hydrogen atom t This reaction can be used to remove an amino group that was ...
... (H 3PO2) to replace the diazonium group with a hydrogen atom t This reaction can be used to remove an amino group that was ...
Carboxylic Acids 2
... 1° alcohols. • To drive equilibrium to the right, one can use an excess of one of the reactants, either the acid or the alcohol as solvent. • Alternatively, the by-product water can be removed by distillation or a dehydrating agent. Esters can be converted back to acids by aqueous acidic hydrolysis: ...
... 1° alcohols. • To drive equilibrium to the right, one can use an excess of one of the reactants, either the acid or the alcohol as solvent. • Alternatively, the by-product water can be removed by distillation or a dehydrating agent. Esters can be converted back to acids by aqueous acidic hydrolysis: ...
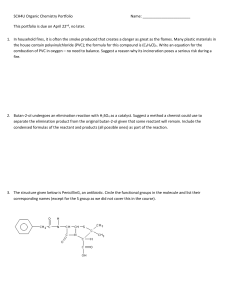
SCH4U Organic Chemistry Portfolio Name: This portfolio is due on
... combustion of PVC in oxygen – no need to balance. Suggest a reason why its incineration poses a serious risk during a fire. ...
... combustion of PVC in oxygen – no need to balance. Suggest a reason why its incineration poses a serious risk during a fire. ...
Year 9 Homework Task 9E-5 Reactions 5-7
... Task: Complete the diagrams. Describe, in words, what happens at each stage of the reaction. Explain what happens during the reaction, using particle diagrams. ...
... Task: Complete the diagrams. Describe, in words, what happens at each stage of the reaction. Explain what happens during the reaction, using particle diagrams. ...
CHE 312 Exam III Review Sheet - Saint Leo University Faculty
... Explain why an aromatic molecule like benzene reacts differently than the corresponding alkene (actually a –triene)? ...
... Explain why an aromatic molecule like benzene reacts differently than the corresponding alkene (actually a –triene)? ...
F324 summary - Macmillan Academy
... Hydrolysis and degradable polymers • Condensation polymers have chemical groups that are vulnerable to chemical attack from either acids or alkalis – polyesters (ester group) and polyamides (amide group). This process is known as hydrolysis and results in the breakdown of the polymer. • Disposing o ...
... Hydrolysis and degradable polymers • Condensation polymers have chemical groups that are vulnerable to chemical attack from either acids or alkalis – polyesters (ester group) and polyamides (amide group). This process is known as hydrolysis and results in the breakdown of the polymer. • Disposing o ...
How to study organic chemistry?
... Bronsted & Lewis theory. Effect of back-bonding , aromaticity, SIR,etc. ...
... Bronsted & Lewis theory. Effect of back-bonding , aromaticity, SIR,etc. ...
Combustion, Addition and Elimination Objective Combustion Example
... Unsaturated hydrocarbon are reacted. Since the site of the double or triple carbon-carbon bond is weaker than the single bond, this bond can break and then we have free bonding electrons where another element or functional group can be added. In general: ...
... Unsaturated hydrocarbon are reacted. Since the site of the double or triple carbon-carbon bond is weaker than the single bond, this bond can break and then we have free bonding electrons where another element or functional group can be added. In general: ...
Reactions of esters:
... The resynthesis of ATP from ADP and Pi is one of the major uses of the chemical energy in the carbohydrates and the fats and oils of our diets. AMINES AND AMIDES: Both amino groups and their protonated form are found in proteins, enzymes and nucleic acids (the chemicals that carry our genes). When a ...
... The resynthesis of ATP from ADP and Pi is one of the major uses of the chemical energy in the carbohydrates and the fats and oils of our diets. AMINES AND AMIDES: Both amino groups and their protonated form are found in proteins, enzymes and nucleic acids (the chemicals that carry our genes). When a ...
Review 3 - Bonham Chemistry
... 21. Industrially, we often need ethanoic acid. The starting material for this product is usually ethane. Show below a series of reactions that would transform ethane to ethanoic acid. ...
... 21. Industrially, we often need ethanoic acid. The starting material for this product is usually ethane. Show below a series of reactions that would transform ethane to ethanoic acid. ...
Esters - Phillips Scientific Methods
... 15. What is the name of the molecule shown in #1? _________________________________ 16. What is the name of the answer you chose from #6? ______________________________ 17. Use your book (Ch 19 p. 503) to help with the following question: The reaction of 2 carboxylic acids produces an anhydride via ...
... 15. What is the name of the molecule shown in #1? _________________________________ 16. What is the name of the answer you chose from #6? ______________________________ 17. Use your book (Ch 19 p. 503) to help with the following question: The reaction of 2 carboxylic acids produces an anhydride via ...
New aniline photocage for carboxylic acids
... on m-hydroxyaniline ethers.[5] We decided to further develop this type of protecting group and expand their application for the protection of carboxylic acids. A systematic study of photochemical reactivity was conducted on o-, m- and p-substituted aniline-based PPGs. We have found out that a range ...
... on m-hydroxyaniline ethers.[5] We decided to further develop this type of protecting group and expand their application for the protection of carboxylic acids. A systematic study of photochemical reactivity was conducted on o-, m- and p-substituted aniline-based PPGs. We have found out that a range ...
organic quiz 2
... Determine the type of reaction that each combination of reactants will undergo and indicate the type of reaction by number in the order (a), (b), (c), and (d). ___, ___, ___, ___. (record your answer on the form provided) Numerical Response #2 The artificial sweetener aspartame is about 180 times as ...
... Determine the type of reaction that each combination of reactants will undergo and indicate the type of reaction by number in the order (a), (b), (c), and (d). ___, ___, ___, ___. (record your answer on the form provided) Numerical Response #2 The artificial sweetener aspartame is about 180 times as ...
Name
... Building Lipid and Protein Molecules Purpose: To model dehydration synthesis reactions resulting in the formation of either protein or lipid molecules. Materials: 14 black carbon atoms 13 red oxygen atoms ...
... Building Lipid and Protein Molecules Purpose: To model dehydration synthesis reactions resulting in the formation of either protein or lipid molecules. Materials: 14 black carbon atoms 13 red oxygen atoms ...
These two compounds are structural isomers, which would have the
... Branches are named as usual (remember to number starting with the functional group) ...
... Branches are named as usual (remember to number starting with the functional group) ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.