Document
... colored complex ions when treated with ceric ammonium nitrate. Other oxygen functional groups (aldehyde, ketone, carboxyl, etc.) do not interfere with the complex formation except as noted in the Limitations. (C. Schiff Test for Aldehydes) The Schiff test (also called the fuchsin-aldehyde test) is b ...
... colored complex ions when treated with ceric ammonium nitrate. Other oxygen functional groups (aldehyde, ketone, carboxyl, etc.) do not interfere with the complex formation except as noted in the Limitations. (C. Schiff Test for Aldehydes) The Schiff test (also called the fuchsin-aldehyde test) is b ...
Exam 2 Fall 2005 Chemsitry 1211
... EXAM #2 Version 1 This exam is twenty five questions long. Each question is worth 4 points. Please read through all of the questions first and ask about anything you do not understand. You will have one hour and 15 minutes to complete this exam. Exams will be picked up at the end of the class period ...
... EXAM #2 Version 1 This exam is twenty five questions long. Each question is worth 4 points. Please read through all of the questions first and ask about anything you do not understand. You will have one hour and 15 minutes to complete this exam. Exams will be picked up at the end of the class period ...
Chapter 9. Addition Reactions of Alkenes
... Copyright© 2012 by Martin Hulce. All rights reserved. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a data base or retrieval system, without the prior permission of the copyrig ...
... Copyright© 2012 by Martin Hulce. All rights reserved. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a data base or retrieval system, without the prior permission of the copyrig ...
An Efficient Synthetic Route to Glycoamino Acid Building Blocks for
... L-aspartic acid R-tert-butyl ester using HOBt/HBTU as coupling agent and subsequent acidic deprotection resulted in the corresponding Fmoc-protected glyco amino acids 4a-e in good overall yields. Optimized yields were obtained with only 1.2-fold excess of amino acid over crude amine, which shows tha ...
... L-aspartic acid R-tert-butyl ester using HOBt/HBTU as coupling agent and subsequent acidic deprotection resulted in the corresponding Fmoc-protected glyco amino acids 4a-e in good overall yields. Optimized yields were obtained with only 1.2-fold excess of amino acid over crude amine, which shows tha ...
Reactions of Hydrocarbons & their functional groups
... • Carbon atom forms fewer bonds to Oxygen or more bonds to Hydrogen • Aldehydes, ketones and carboxyliic acids can be “reduced” to alcohols • Alkenes and alkynes can be reduced to become alkanes • Occurs in the presence of reducing agents such as LiAlH4, and H2/Pt where Hydrogen [H] is added ...
... • Carbon atom forms fewer bonds to Oxygen or more bonds to Hydrogen • Aldehydes, ketones and carboxyliic acids can be “reduced” to alcohols • Alkenes and alkynes can be reduced to become alkanes • Occurs in the presence of reducing agents such as LiAlH4, and H2/Pt where Hydrogen [H] is added ...
Intro to Organic Reactions
... Ethanol is a PRIMARY ALCOHOL. It is oxidized to an ALDEHYDE and then to an ACID. ...
... Ethanol is a PRIMARY ALCOHOL. It is oxidized to an ALDEHYDE and then to an ACID. ...
effective: september 2003 curriculum guidelines
... Aldehydes and Ketones and Addition Reactions to the Carbonyl Group: Nomenclature, Physical Propeerties, Synthesis of Aldehydes and Ketones, Nucleophilic Addition to Carbonyl Group, Addition of Water, and Alcohols, Hemiacetal and Hemiketal Formation, Acetal and Ketal Formation, Ammonia Derivatives, W ...
... Aldehydes and Ketones and Addition Reactions to the Carbonyl Group: Nomenclature, Physical Propeerties, Synthesis of Aldehydes and Ketones, Nucleophilic Addition to Carbonyl Group, Addition of Water, and Alcohols, Hemiacetal and Hemiketal Formation, Acetal and Ketal Formation, Ammonia Derivatives, W ...
IR Spectroscopy of Esters - Purdue College of Science
... b. Add 10 drops of one of the alcohols c. Add 2 drops of concentrated sulfuric acid 3. Place test tube in water bath for about 5 minutes 4. To smell the esters, do not inhale the odor from the test tube. Instead either pour part of the solution in a beaker of about 100 mL ofwater and waft the vapors ...
... b. Add 10 drops of one of the alcohols c. Add 2 drops of concentrated sulfuric acid 3. Place test tube in water bath for about 5 minutes 4. To smell the esters, do not inhale the odor from the test tube. Instead either pour part of the solution in a beaker of about 100 mL ofwater and waft the vapors ...
Chemistry Final Test
... (B) Heat can disrupt tertiary structure. (C) Nonpolar groups tend to face the outside of a protein in an aqueous solution. (D) Ionized amino acid side chains can form salt bridges within a protein. (E) Disulfide bonds provide strong intrachain interactions. 6-19、Which statement is true? (A) Each gen ...
... (B) Heat can disrupt tertiary structure. (C) Nonpolar groups tend to face the outside of a protein in an aqueous solution. (D) Ionized amino acid side chains can form salt bridges within a protein. (E) Disulfide bonds provide strong intrachain interactions. 6-19、Which statement is true? (A) Each gen ...
Amino acids joined by peptide bonds
... Phenylalanine is a building block of proteins called an amino acid that is obtained through the diet. It is found in all proteins and in some artificial sweeteners. PKU occurs because the affected individual lacks the enzyme phenylalanine hydroxylase. If PKU is not treated, phenylalanine (substrate) ...
... Phenylalanine is a building block of proteins called an amino acid that is obtained through the diet. It is found in all proteins and in some artificial sweeteners. PKU occurs because the affected individual lacks the enzyme phenylalanine hydroxylase. If PKU is not treated, phenylalanine (substrate) ...
Chemistry 218, Winter 2007 Exam 2 Name: 1.
... 5. Draw an energy diagram of the molecular orbitals of oxazole (you do not need to draw the molecular orbitals, just their relative energies). From your diagram, determine if the molecule is aromatic or anti-aromatic. (8 pts.) O N ...
... 5. Draw an energy diagram of the molecular orbitals of oxazole (you do not need to draw the molecular orbitals, just their relative energies). From your diagram, determine if the molecule is aromatic or anti-aromatic. (8 pts.) O N ...
Organic Reactions 2.1- 2.3 - mccormack-sch4u-2013
... • Carbon atom forms fewer bonds to Oxygen or more bonds to Hydrogen • Aldehydes, ketones and carboxyliic acids can be “reduced” to alcohols • Alkenes and alkynes can be reduced to become alkanes • Occurs in the presence of reducing agents such as LiAlH4, and H2 where Hydrogen [H] is added ...
... • Carbon atom forms fewer bonds to Oxygen or more bonds to Hydrogen • Aldehydes, ketones and carboxyliic acids can be “reduced” to alcohols • Alkenes and alkynes can be reduced to become alkanes • Occurs in the presence of reducing agents such as LiAlH4, and H2 where Hydrogen [H] is added ...
Chem 30CL - Lecture 1c - UCLA Chemistry and Biochemistry
... • The yields are moderate (77 % for the reaction above) due to the increased water solubility of the products ...
... • The yields are moderate (77 % for the reaction above) due to the increased water solubility of the products ...
Microsoft Word
... reactions: Guanidines are of considerable interest in biology and synthetic organic chemistry. Although there are many biological applications of guanidines, relatively few studies have examined their use in organic synthesis. Guanidines can be used as catalysts in carbon-carbon bond forming reactio ...
... reactions: Guanidines are of considerable interest in biology and synthetic organic chemistry. Although there are many biological applications of guanidines, relatively few studies have examined their use in organic synthesis. Guanidines can be used as catalysts in carbon-carbon bond forming reactio ...
投影片 1
... The positive charge (+) is placed at the carbon attached to the E class function group (e.g.,=O,-OH, -Br) Consonant pattern: Positives charges are placed at carbon atoms bonded to the E class groups ...
... The positive charge (+) is placed at the carbon attached to the E class function group (e.g.,=O,-OH, -Br) Consonant pattern: Positives charges are placed at carbon atoms bonded to the E class groups ...
Pre Ch15 HW
... bonds, and chains, which results in the great structural diversity of organic compounds (§15.1) 2. How carbon's atomic properties give rise to its ability to bond to various heteroatoms, which creates regions of charge imbalance that result in functional groups (§15.1) 3. Structures and names of alk ...
... bonds, and chains, which results in the great structural diversity of organic compounds (§15.1) 2. How carbon's atomic properties give rise to its ability to bond to various heteroatoms, which creates regions of charge imbalance that result in functional groups (§15.1) 3. Structures and names of alk ...
chapter 2: reactions of organic compounds
... • Carbon atom forms fewer bonds to Oxygen or more bonds to Hydrogen • Aldehydes, ketones and carboxyliic acids can be “reduced” to alcohols • Alkenes and alkynes can be reduced to become alkanes • Occurs in the presence of reducing agents such as LiAlH4, and H2 where Hydrogen [H] is added ...
... • Carbon atom forms fewer bonds to Oxygen or more bonds to Hydrogen • Aldehydes, ketones and carboxyliic acids can be “reduced” to alcohols • Alkenes and alkynes can be reduced to become alkanes • Occurs in the presence of reducing agents such as LiAlH4, and H2 where Hydrogen [H] is added ...
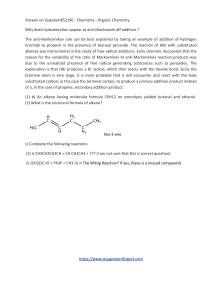
Answer on Question#52196 - Chemistry
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
AMIDES & AMINES
... • Can be thought of as ammonia (NH3), with one, two or all three of its hydrogens substituted by alkyl groups • The number of substituted alkyl groups allow for primary (10), secondary (20), and tertiary (30) sub-classifications (similar to alcohols) ...
... • Can be thought of as ammonia (NH3), with one, two or all three of its hydrogens substituted by alkyl groups • The number of substituted alkyl groups allow for primary (10), secondary (20), and tertiary (30) sub-classifications (similar to alcohols) ...
unit 4 revision checklist - A
... = pKa at half-neutralisation, and calculate the pH during titrations involving alkalis added to acids, including after excess alkali has been added n) Select a suitable indicator for a given titration given pKin values, and explain the choice of indicator o) Recall that polybasic acids form more tha ...
... = pKa at half-neutralisation, and calculate the pH during titrations involving alkalis added to acids, including after excess alkali has been added n) Select a suitable indicator for a given titration given pKin values, and explain the choice of indicator o) Recall that polybasic acids form more tha ...
Oxidation of Ethanol, Esters, Polymerization, Amino
... solution containing the dichromate (VI) ions is reduced to a green solution containing chromium (III) ions. The electron-half-equation for this reaction is ...
... solution containing the dichromate (VI) ions is reduced to a green solution containing chromium (III) ions. The electron-half-equation for this reaction is ...
Carboxylic Acids
... Electron withdrawing groups can increase acid strength by weakening the OH bond and stabilizing the acid anion. The positive inductive effect of E-groups is very small through more than two or three carbon-carbon bonds. Electron donating groups reduce the partially positive charge of carboxyl carbon ...
... Electron withdrawing groups can increase acid strength by weakening the OH bond and stabilizing the acid anion. The positive inductive effect of E-groups is very small through more than two or three carbon-carbon bonds. Electron donating groups reduce the partially positive charge of carboxyl carbon ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.