* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download AMIDES & AMINES
Homoaromaticity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Bottromycin wikipedia , lookup
Hydroformylation wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
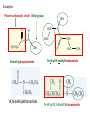
AMIDES & AMINES Introduction • So far, we’ve dealt with compounds containing C, H, and O atoms. • Many naturally produced compounds also contain nitrogen. • Organic compounds that contain nitrogen can either be called amines or amides AMINES • Can be thought of as ammonia (NH3), with one, two or all three of its hydrogens substituted by alkyl groups • The number of substituted alkyl groups allow for primary (10), secondary (20), and tertiary (30) sub-classifications (similar to alcohols) Common Names for Amines: Text method 1. Identify the largest alkyl group attached to the nitrogen atom as the parent alkane 2. Replace the -e at the end of the parent alkane with the new ending –amine. Include a position number if required Butan-1-amine IUPAC Names for 20 and 30 Amines • The IUPAC names for 20 and 30 amines include the N- prefix to denote the substituted groups on the N atom of the amino group. • The larger alkyl group determines the main chain • If the structure is a 30 amine, one group must be considered the main chain, while the other groups are considered branches • Additional side chains are listed in alphabetical order as normal N-methylbutan-1-amine N,N-dimethylmethanamine 5-chloro-N,N-dimethylhexan-3-amine Properties of Amines • N-C and N-H bonds are polar, thereby allowing for dipole-dipole and London forces. • In addition, N-H bonds allow for hydrogen bonding. Note that N-H hydrogen bonds are weaker than O-H hydrogen bonds Preparing Amines: Substitution Reactions • Amines are prepared by the reaction of ammonia with an alkyl halide (let X represent any halogen) AMIDES Structure and Properties • Contain the amide linkage as their functional group. O R C R'' N R' •Structurally similar to esters. •Amide linkage is very significant in biological systems as the forming and breaking of these bonds give specificity to proteins. Naming Amides • Locate the part of the amide that contains the C=O group. Name the parent carboxylic acid that this part derives from. The C=O group is always given position number 1 • Replace the –oic acid ending of the name of the parent acid with the suffic –amide Ethanoic acid 2 1 ethanamide Amide Branches • If the amide is a 20 amide, name the alkyl group and give it location letter N- to indicate that it is bonded to the nitrogen atom • If the amide is a 30 amide, place the alkyl groups in alphabetical order. Use location letter N- before each group to indicate that it is bonded to the nitrogen. If the two groups are identical, use N,N- Examples Parent carboxylic chain Alkyl group H3CH2C O H C N CH3 H2C N H3C CH3 N-methyl propanamide N,N-diethylethanamide C O CH2 H2C CH3 N-ethyl-N-methyl butanamide N-ethyl-N,3-dimethylbutanamide Preparation • Amides are made through a condensation reaction of a carboxylic acid and a primary or secondary amine. • Tertiary amines can not be used as they do not contain a hydrogen. carboxylic acid + O + H OH propanoic acid H2SO4 amide + water H2SO4 N C H3CH2C 1°or 2°amine CH3 H methylamine methanamine H3CH2C O H C N + H2O CH3 N-methylpropanamide Amino Acids O R CH NH2 C OH carbon • Amino acids are bifunctional because they contain both an amino group (NH2) and a carboxyl group (COOH). • Depending on what they are mixed with, amino acids can act as either an acid or a base. • The amino and carboxyl groups are attached to the same carbon ( carbon). • The 20 different amino acids only differ in their side chains (R group).