- KCN K+ R KOH + H2O
... Remember, RBr ⇒ ROH; and we have seen that RCHO or R2CO ⇒ R”CH2OH or R”2CHOH (oxidation of aldehydes and ketones) Which starting materials would you use to prepare PhCH=C(CH3)2? PhCHO and (CH3)2CHBr versus PhCH2Br and (CH3)2CO? How would you prepare PhCH2Br from PhCOOMe? How would you prepare PhCHO ...
... Remember, RBr ⇒ ROH; and we have seen that RCHO or R2CO ⇒ R”CH2OH or R”2CHOH (oxidation of aldehydes and ketones) Which starting materials would you use to prepare PhCH=C(CH3)2? PhCHO and (CH3)2CHBr versus PhCH2Br and (CH3)2CO? How would you prepare PhCH2Br from PhCOOMe? How would you prepare PhCHO ...
6 Biological Molecules-S - Elmwood Park Memorial Middle School
... of four classes of organic (carbon-based) compounds—carbohydrates, lipids, proteins, and nucleic acids. These organic molecules are the building blocks of all living things, and are responsible for most of the structure and functions of the body, including energy storage, insulation, growth, repair, ...
... of four classes of organic (carbon-based) compounds—carbohydrates, lipids, proteins, and nucleic acids. These organic molecules are the building blocks of all living things, and are responsible for most of the structure and functions of the body, including energy storage, insulation, growth, repair, ...
Exam 2 Review A
... Carbocation structure and trends in stability. Hyperconjugation and inductive effects. Carbocations can do three things: 1. React with nucleophiles, 2. -eliminate (lose H+) to form an alkene, 3. undergo skeletal rearrangements via 1,2-hydride shifts or 1,2-methanide shifts. [we will defer discussio ...
... Carbocation structure and trends in stability. Hyperconjugation and inductive effects. Carbocations can do three things: 1. React with nucleophiles, 2. -eliminate (lose H+) to form an alkene, 3. undergo skeletal rearrangements via 1,2-hydride shifts or 1,2-methanide shifts. [we will defer discussio ...
12SN-23-10 OBJECTIVE: Identify how alcohols are classified and
... Identify how alcohols are classified and named. Predict how the solubility of an alcohol varies with the length of its carbon chain. Name the reactions of alkenes that may be used to introduce functional groups. Construct the general structure of an ether and describe how ethers are named. Identify ...
... Identify how alcohols are classified and named. Predict how the solubility of an alcohol varies with the length of its carbon chain. Name the reactions of alkenes that may be used to introduce functional groups. Construct the general structure of an ether and describe how ethers are named. Identify ...
C - Milwaukie High
... Organic Reactions combustion of hydrocarbons OR compounds w/only C, H, and O: products are…CO2 and H2O Write the equation for the complete combustion of 2-methyl-2-pentene. C6H12 + 9 O2 6 CO2 + 6 H2O ...
... Organic Reactions combustion of hydrocarbons OR compounds w/only C, H, and O: products are…CO2 and H2O Write the equation for the complete combustion of 2-methyl-2-pentene. C6H12 + 9 O2 6 CO2 + 6 H2O ...
Annexure `CD-01` L T P/S SW/FW TOTAL CREDIT UNITS 3 1 4 0 6
... List of Experiments: 1. Determination of concentration of a given sucrose solution using a Polarimeter. 2. Estimation of reducing and non-reducing sugars by Fehling’s method. 3. Estimation of carbohydrate by anthrone method spectrometrically. 4. Derivatization of Glucose and fructose to osazone 5. E ...
... List of Experiments: 1. Determination of concentration of a given sucrose solution using a Polarimeter. 2. Estimation of reducing and non-reducing sugars by Fehling’s method. 3. Estimation of carbohydrate by anthrone method spectrometrically. 4. Derivatization of Glucose and fructose to osazone 5. E ...
File
... • competes with the elimination reaction (dehydration) but this reaction is favored using a large excess of concentrated acid (HCl and zinc chloride) • we can distinguish between 1°, 2° and 3° alcohols by their different rates of reaction using the Lucas Test • order of reactivity: – tertiary > seco ...
... • competes with the elimination reaction (dehydration) but this reaction is favored using a large excess of concentrated acid (HCl and zinc chloride) • we can distinguish between 1°, 2° and 3° alcohols by their different rates of reaction using the Lucas Test • order of reactivity: – tertiary > seco ...
Polyesters are condensation polymers.
... Note that sometimes other molecules (HCl for eg) are lost in other condensation reactions. It is the elimination of a molecule which makes it a condensation reaction, not the loss of water. ...
... Note that sometimes other molecules (HCl for eg) are lost in other condensation reactions. It is the elimination of a molecule which makes it a condensation reaction, not the loss of water. ...
Study Questions - Labs - Department of Plant Biology, Cornell
... 3.1 Carbon atoms found on earth were synthesized: ...
... 3.1 Carbon atoms found on earth were synthesized: ...
CHEMISTRY
... (2) The system is at equilibrium (3) A catalyst is added (4) The reactants are initially mixed 19. For the reaction CI2(g) + 2 NO (g) 2 NOCI (g) doubling the concentration of both reactants increases the rate by a factor of eight. If only the concentration of CI2 is doubled, the rate increases by ...
... (2) The system is at equilibrium (3) A catalyst is added (4) The reactants are initially mixed 19. For the reaction CI2(g) + 2 NO (g) 2 NOCI (g) doubling the concentration of both reactants increases the rate by a factor of eight. If only the concentration of CI2 is doubled, the rate increases by ...
Exam 2 Review A
... Carbocation structure and trends in stability. Hyperconjugation and inductive effects. Carbocations can do three things: 1. React with nucleophiles, 2. -eliminate (lose H+) to form an alkene, 3. undergo skeletal rearrangements via 1,2-hydride shifts or 1,2-methanide shifts. [we will defer discussio ...
... Carbocation structure and trends in stability. Hyperconjugation and inductive effects. Carbocations can do three things: 1. React with nucleophiles, 2. -eliminate (lose H+) to form an alkene, 3. undergo skeletal rearrangements via 1,2-hydride shifts or 1,2-methanide shifts. [we will defer discussio ...
Elimination reactions under acidic conditions
... 4. In each of these reactions, there is more than one alkene that could form. Draw out all possible products, and then circle the one you would predict to be the major product. (Don’t worry about cis/trans isomers.) ...
... 4. In each of these reactions, there is more than one alkene that could form. Draw out all possible products, and then circle the one you would predict to be the major product. (Don’t worry about cis/trans isomers.) ...
C h e m g u i d e ... ALCOHOLS: ESTERIFICATION
... (You first need to work out the structure of the alcohol from its name: CH3CHCH CH OH ...
... (You first need to work out the structure of the alcohol from its name: CH3CHCH CH OH ...
Test3
... Acids are defined as compounds that produce H+ ions in water solution. Bases are defined as compounds that produce OH- ions in water solution. Arrhenius theory only applies to reactions in aqueous solution. Acids are defined as compounds that produce OH- ions in water solution. ...
... Acids are defined as compounds that produce H+ ions in water solution. Bases are defined as compounds that produce OH- ions in water solution. Arrhenius theory only applies to reactions in aqueous solution. Acids are defined as compounds that produce OH- ions in water solution. ...
Chapter 24. Amines
... clear-cut coupling to neighboring C–H hydrogens In D2O exchange of N–D for N–H occurs, and the N– ...
... clear-cut coupling to neighboring C–H hydrogens In D2O exchange of N–D for N–H occurs, and the N– ...
organic lab questions
... Write descriptive organic equations for all reactions. If no reaction occurred, write N.R. for the products of the reaction. Under each alcohol, write the common name of the alcohol. Under each carboxylic acid write the I.U.P.A.C. name of the carboxylic acid. Finally, write the name of the ester und ...
... Write descriptive organic equations for all reactions. If no reaction occurred, write N.R. for the products of the reaction. Under each alcohol, write the common name of the alcohol. Under each carboxylic acid write the I.U.P.A.C. name of the carboxylic acid. Finally, write the name of the ester und ...
슬라이드 1
... Existing substituent groups such as CH3, OCH3, and +NMe3 exert a directive effect, often resulting in a major amount of the meta substitution product. ...
... Existing substituent groups such as CH3, OCH3, and +NMe3 exert a directive effect, often resulting in a major amount of the meta substitution product. ...
lec-2- 211(ES +Add)
... Benzene is treated with a mixture of concentrated nitric acid and concentrated sulphuric acid at a temperature not exceeding 50°C. As temperature increases there is a greater chance of getting more than one nitro group, -NO2, substituted onto the ring. Nitrobenzene is formed. H2SO4 ...
... Benzene is treated with a mixture of concentrated nitric acid and concentrated sulphuric acid at a temperature not exceeding 50°C. As temperature increases there is a greater chance of getting more than one nitro group, -NO2, substituted onto the ring. Nitrobenzene is formed. H2SO4 ...
Current Research Click Here
... Reagents for the oxidation of alcohols are often undesirable from an environmental point of view. Reagents such as PCC and the chemicals used in the Swern oxidation are not environmentally friendly. A recent report in the literature uses a Pd resin with the same functional group as the oxidation cat ...
... Reagents for the oxidation of alcohols are often undesirable from an environmental point of view. Reagents such as PCC and the chemicals used in the Swern oxidation are not environmentally friendly. A recent report in the literature uses a Pd resin with the same functional group as the oxidation cat ...
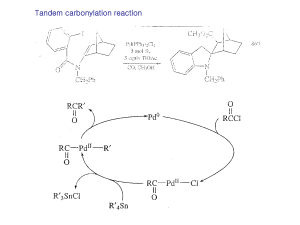
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.