* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ORGANIC
Survey
Document related concepts
Transcript
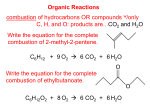
ORGANIC CHEMISTRY FUNCTIONAL GROUPS HYDROCARBONS Carbon being group IV or 14 is able to form 4 strong covalent bonds with other elements such as hydrogen, or itself, to produce long chains Organic compounds can be straight or branched Classes of hydrocarbons 2 main classes Aromatic – benzene Aliphatic – Alkane, Alkene Alkyne If one of the hydrogen atoms in an alkane is missing then it is called an alkyl group Functional groups Functional groups are basic units that exist on the carbon chain. Alcohol -ol R-OH CH3OH : methanol Aldehyde -al RCHO C2H5CHO : propanal Ketone -one RCOR CH3COC2H5 : butanone Carboxylic acid -oic acid RCOOH CH3COOH : ethanoic acid Esters -yl -oate RCOOR HCOOC2H5 : ethyl methanoate Amine -amine RNH2 C2H5NH2 ethanamine Naming Identify functional group on the chain Identify the longest chain containing the functional group Number the carbons in the chain to give the main functional group the lowest number Number all functional groups in order List alphebetically naming Lowest number rank Amine amino Alcohol hydroxy Aldehyde Ketone Carboxylic acid Isomers – same formula different arrangement Geometric – are compounds with the same molecular formula with alkyl or functional groups to the same numbered carbon atoms but arranged differently in space – only exist where there is a double bond cis and trans Structural Naming Alcohols Primary ROH Secondary R Tertiary - OH - R R R - OH - R Reactions Alkanes combustion and substitution Alkenes combustion and addition combustion, with group 1 metals, oxidation, esterfication Alcohols Alcohols React with Na to produce alk-oxide and hydrogen gas 10 and 20 oxidise with acidified KMnO4 or acidified K2Cr2O7 which both lose their colour Primary oxidise partially to an aldehyde and fully to a carboxylic acid. Secondary oxidise to ketones Esters Alcohols react with carboxylic acids in the presence of H2SO4 to produce Esters Propanoic acid + methanol methyl propanoate Smelly – mainly a fruity smell Hydrolysis in acid conditions - alcohol and CA Hydrolysis in basic conditions - soap and alcohol Intermolecular – alcohols, aldehydes, ketones, carboxylic acids. Polar polar – anes, enes ynes, benzene, esters. Non Soap Produced by saponification of fats and oils using NaOH. Fats and oils are esters [Triglycerides] of long chain carboxylic acids – fatty acids. The sodium salt of the long chain acid is called soap. C17H35COONa – sodium stearate Cleaning action of soaps - Ionization of the molecule to give a charged end C17H35COONa C17H35COO- + Na+ stearate ion ------------O- Soap The stearate ion has one polar end with a –ve charge which is attracted to the water and one non polar end which is attracted to the non-polar molecules ------------O Hydrophobic Hydrophilic Dislodge and disperse nonpolar molecules like oil and dirt. Polymerisation process of joining short molecules – monomers- into long chain molecules Two types Addition And Condensation polymerization The Addition Involves a double bond and Catalyst a carbon = carbon double bond, the π bond is broken to link up the monomers using a catalyst In n CH2=CH catalyst [- CH2- CH - ] l = opens l R R Condensation This involves a reaction between two different functional groups in which a molecule of water is eliminated and the two groups link together Usually involves two different monomers The most common types of condensation polymer occurs between a carboxylic acid and an alcohol or amine groups condensation Involves two functional groups – one a carboxylic acid the other an alcohol or amine The carboxylic acid needs to be a di-oic acid and the other group need to be di-ol or di-amine condensation n(HOOC-R-COOH) + n(HO-R-OH) n(-OC-R-COO-R-O) + 2nH2O n(-OC-R-CO / O-R-O ) OH / H H HO Condensation HOOC-(CH2)4-COOH + H2N-(CH2)6-NH2 -[OC-(CH2)4-COHN-(CH2)6-NH] + 2nH2O Hexandioic acid and 1,6 Hexandiamine produces Nylon 66 Amino acids Amino acids are compounds that contain an amine group and a carboxylic acid group. • For amino acids these two functional groups are bonded to the same C atom. Amino acids have both an acidic and a basic functional group.