Chapter 1 Structure and Bonding
... a. Electropositive carbonyl carbon pulls electrons away from O—H bond b. Resulting carboxylate anion is stabilized by resonance c. pKa’s usually between 4 and 5 O R ...
... a. Electropositive carbonyl carbon pulls electrons away from O—H bond b. Resulting carboxylate anion is stabilized by resonance c. pKa’s usually between 4 and 5 O R ...
Chap Thirteen: Alcohols
... inversion of configuration incomplete iii. SN2 reaction With phosphorus trihalides PBr3 or PCl3 or PCl5 or P° and I2 to form alkyl halides o Mechanism/ no rearrangement/ inversion of configuration e. Alkyl tosylates (sulfonate esters) by reaction of ROH with sulfonyl chlorides i. Mechanism/ retentio ...
... inversion of configuration incomplete iii. SN2 reaction With phosphorus trihalides PBr3 or PCl3 or PCl5 or P° and I2 to form alkyl halides o Mechanism/ no rearrangement/ inversion of configuration e. Alkyl tosylates (sulfonate esters) by reaction of ROH with sulfonyl chlorides i. Mechanism/ retentio ...
Outline_CH13_Klein
... inversion of configuration incomplete iii. SN2 reaction With phosphorus trihalides PBr3 or PCl3 or PCl5 or P° and I2 to form alkyl halides o Mechanism/ no rearrangement/ inversion of configuration e. Alkyl tosylates (sulfonate esters) by reaction of ROH with sulfonyl chlorides i. Mechanism/ retentio ...
... inversion of configuration incomplete iii. SN2 reaction With phosphorus trihalides PBr3 or PCl3 or PCl5 or P° and I2 to form alkyl halides o Mechanism/ no rearrangement/ inversion of configuration e. Alkyl tosylates (sulfonate esters) by reaction of ROH with sulfonyl chlorides i. Mechanism/ retentio ...
CET MODEL QUESTION PAPER 1. Set of quantum numbers (n, /, m
... 12. For an endothermic reaction, where .:\11 represents the enthalpy of the reaction in KJ / mole, the minimum value for the energy of activation will be 1] less than ∆H 2] zero 3] more than ∆H 4] equal to ∆H 13. Zone refining is a method to obtain I] very high temperature 2] ultra pure Al 14. An or ...
... 12. For an endothermic reaction, where .:\11 represents the enthalpy of the reaction in KJ / mole, the minimum value for the energy of activation will be 1] less than ∆H 2] zero 3] more than ∆H 4] equal to ∆H 13. Zone refining is a method to obtain I] very high temperature 2] ultra pure Al 14. An or ...
Chem 3.5 #6 Amines
... Amines with low molecular mass are soluble in water. Explain why they can dissolve in water. ...
... Amines with low molecular mass are soluble in water. Explain why they can dissolve in water. ...
Senior Science topics Programme
... The programme consists of five parts: 1. Hydrogenation of alkenes This part introduces to students the characteristic reaction of alkenes, addition reaction, as exemplified by the hydrogenation of ethene. The process in which the reaction is speeded up by the presence of a catalyst is illustrated by ...
... The programme consists of five parts: 1. Hydrogenation of alkenes This part introduces to students the characteristic reaction of alkenes, addition reaction, as exemplified by the hydrogenation of ethene. The process in which the reaction is speeded up by the presence of a catalyst is illustrated by ...
CH 115 Exam 2 - UAB General Chemistry Supplemental Instruction
... Assume the chemical equations on this exam are NOT balanced unless stated otherwise. 1. Balance the equation and give the stoichiometric coefficient for HCl ...
... Assume the chemical equations on this exam are NOT balanced unless stated otherwise. 1. Balance the equation and give the stoichiometric coefficient for HCl ...
Q1. Give I.U.P.A..C Name of the following Organic Compound. 1 CH
... (a) Are all the five bonds in Pcl5 molecule equivalent? Justify your answer. (b) H3PO3 is diprotic? (c) On addition of Ozone gas to KI solution violet vapours are obtained. Q 24 the following results have been obtained during the Kinetic studies of the reaction. 3 2A + B Experiment ...
... (a) Are all the five bonds in Pcl5 molecule equivalent? Justify your answer. (b) H3PO3 is diprotic? (c) On addition of Ozone gas to KI solution violet vapours are obtained. Q 24 the following results have been obtained during the Kinetic studies of the reaction. 3 2A + B Experiment ...
... Johnson Matthey have published an informative 82-page brochure, “The Catalyst Technical Handbook”, which covers the use of catalysts for chemical reactions important in industrial synthesis. The handbook recommends platinum group metal homogeneous, heterogeneous and FibreCatm anchored homogeneous ca ...
Redox Reactions
... Hydride reagents provide H- as a nucleophile, therefore they only react with polar substrates containing an electrophile. They do not react with alkenes or alkynes, so they provide additional selectivity between the functional groups. The reaction mechanism involves the nucleophilic attack of the ca ...
... Hydride reagents provide H- as a nucleophile, therefore they only react with polar substrates containing an electrophile. They do not react with alkenes or alkynes, so they provide additional selectivity between the functional groups. The reaction mechanism involves the nucleophilic attack of the ca ...
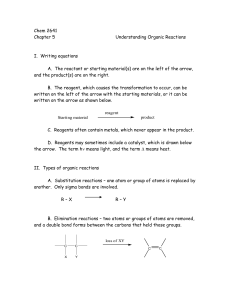
Chem 2641 Chapter 5 Understanding Organic Reactions I. Writing
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
... The initiation stage – Cl2 reacts with the uv light to form Cl. Propagation stage – The Cl. reacts with CH4 to form CH3. and HCl The CH3. reacts with Cl2 to form CH3Cl and Cl. Termination stage – any two radicals can combine to form a stable product. ...
C h e m g u id e –... AMINO ACIDS: INTRODUCTION
... therefore transfers from the COOH group to the NH2 group giving the zwitterion NH3+ R-CH-COOb) There will be ionic bonds between the separate molecules, and these take more energy to break that other intermolecular forces. c) There are attractions between the very polar water molecules and the zwitt ...
... therefore transfers from the COOH group to the NH2 group giving the zwitterion NH3+ R-CH-COOb) There will be ionic bonds between the separate molecules, and these take more energy to break that other intermolecular forces. c) There are attractions between the very polar water molecules and the zwitt ...
Lecture 2 - UCLA Chemistry and Biochemistry
... For both isomers the conformer on the left is the major conformer because it is energetically lower (DG‡(axialequatorial): CH3: 7.28 kJ/mol, OH: 3.90 kJ/mol). The leaving group has to be in axial position for the elimination to occur. The cis isomer of the protonated alcohol reacts out its major con ...
... For both isomers the conformer on the left is the major conformer because it is energetically lower (DG‡(axialequatorial): CH3: 7.28 kJ/mol, OH: 3.90 kJ/mol). The leaving group has to be in axial position for the elimination to occur. The cis isomer of the protonated alcohol reacts out its major con ...
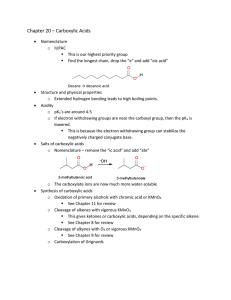
Chapter 20 - people.vcu.edu
... o What would happen if you put the following reagents into a vessel? ...
... o What would happen if you put the following reagents into a vessel? ...
Benzocaine Synthesis via Esterification
... pipette to transfer the acid. A precipitate is expected to form. Attach a reflux condenser to the flask, secure the apparatus with clamps, and heat the mixture at a gentle reflux for 60-75 minutes using a heating mantle and Variac power controller. The solid should dissolve as it undergoes reaction. ...
... pipette to transfer the acid. A precipitate is expected to form. Attach a reflux condenser to the flask, secure the apparatus with clamps, and heat the mixture at a gentle reflux for 60-75 minutes using a heating mantle and Variac power controller. The solid should dissolve as it undergoes reaction. ...
CfE Higher Chemistry Homework Unit 2: Natures Chemistry
... What term can be applied to aspirin but not oil of wintergreen? 4. A student carried out four tests on ethanol and ethanoic acids to compare the properties of the two homologous series, alcohols and carboxylic acids. a. Choose one test in which ethanol and ethanoic acid will give different results a ...
... What term can be applied to aspirin but not oil of wintergreen? 4. A student carried out four tests on ethanol and ethanoic acids to compare the properties of the two homologous series, alcohols and carboxylic acids. a. Choose one test in which ethanol and ethanoic acid will give different results a ...
Synthesis and Characterization of N- Cbz L- Aspartic acid β
... Amino acids are critical to life, and have many functions in metabolism. One particularly important function is to serve as the building blocks of proteins, which are linear chains of amino acids. This article discusses the synthesis of useful amino acids from aspartic acid as a starting material. T ...
... Amino acids are critical to life, and have many functions in metabolism. One particularly important function is to serve as the building blocks of proteins, which are linear chains of amino acids. This article discusses the synthesis of useful amino acids from aspartic acid as a starting material. T ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.