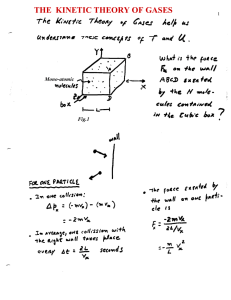
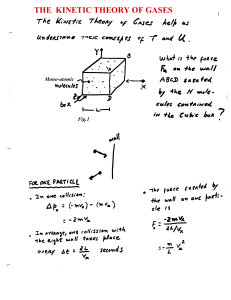
the kinetic theory of gases
... reached when the pressure at both side is the same. According to expression (1), P = (2/3) (N/V) <½ mv2>; thus a condition of MECHANICAL EQUILIBRIUM, would imply, (2/3) (N1/V1) <½ m1v12> = (2/3) (N2/V2) <½ m2v22> It turns out, as argued in the Feynman lectures Vol-1, chapter 39), this condition is n ...
... reached when the pressure at both side is the same. According to expression (1), P = (2/3) (N/V) <½ mv2>; thus a condition of MECHANICAL EQUILIBRIUM, would imply, (2/3) (N1/V1) <½ m1v12> = (2/3) (N2/V2) <½ m2v22> It turns out, as argued in the Feynman lectures Vol-1, chapter 39), this condition is n ...
Article3-Dirac - Inframatter Research Center
... even at z near 137 this is less than a half percent of the magnitude of the masscentripetal effect just calculated (so can probably be ignored for now). Entanglement effects have also been theorized, but again only at extreme energies which an orbiting electron can’t produce. This argument treats th ...
... even at z near 137 this is less than a half percent of the magnitude of the masscentripetal effect just calculated (so can probably be ignored for now). Entanglement effects have also been theorized, but again only at extreme energies which an orbiting electron can’t produce. This argument treats th ...
slides introducing IR/Raman of proteins
... – In practice, stronger bonds have sharper (more curvature) potential energy curves, result: higher k, and higher frequency. and µ is the reduced mass [m1m2 / (m1+m2)]. ...
... – In practice, stronger bonds have sharper (more curvature) potential energy curves, result: higher k, and higher frequency. and µ is the reduced mass [m1m2 / (m1+m2)]. ...
chapter 5 thermochemistry
... Another common energy unit is the calorie (cal), which was originally defined as the quantity of energy necessary to increase the temperature of 1 g of water by 1°C: When we study thermodynamic properties, we define a specific amount of matter as the system. Everything outside the system is the surr ...
... Another common energy unit is the calorie (cal), which was originally defined as the quantity of energy necessary to increase the temperature of 1 g of water by 1°C: When we study thermodynamic properties, we define a specific amount of matter as the system. Everything outside the system is the surr ...
Applied Physics - Revision World
... Angular velocity: the angle of a circle (arc) mapped out by a ...
... Angular velocity: the angle of a circle (arc) mapped out by a ...
PROGRAM ON PHYSICS MECHANICS Kinematics. Mechanical
... Electrostatics. Electrization bodies. The Electric charge. Interaction of charges. An elementary electric charge. The law of preservation of an electric charge. The Coulomb law. Electric field. Intensity of electric field. Electric field of a dot charge. Potentiality of an electrostatic field. A pot ...
... Electrostatics. Electrization bodies. The Electric charge. Interaction of charges. An elementary electric charge. The law of preservation of an electric charge. The Coulomb law. Electric field. Intensity of electric field. Electric field of a dot charge. Potentiality of an electrostatic field. A pot ...
Conservation of Energy and Momentum
... taken away (Q) and the work done by or on system (W): U = ____________________ 36. ___________________________ is a quantity that measures the disorder of a system and this quantity is larger for a more disordered system. 37. Most processes tend to decrease the order of a system over time. Energy l ...
... taken away (Q) and the work done by or on system (W): U = ____________________ 36. ___________________________ is a quantity that measures the disorder of a system and this quantity is larger for a more disordered system. 37. Most processes tend to decrease the order of a system over time. Energy l ...
Part I – Mechanics
... change a substance from a solid to liquid or liquid to a solid at constant temperature and pressure vi. Heat of Vaporization – energy per unit mass transferred in order to change a substance from a liquid to a vapor or from a vapor to a liquid at constant temperature and pressure. vii. Because gas m ...
... change a substance from a solid to liquid or liquid to a solid at constant temperature and pressure vi. Heat of Vaporization – energy per unit mass transferred in order to change a substance from a liquid to a vapor or from a vapor to a liquid at constant temperature and pressure. vii. Because gas m ...
File - Prairie Science
... heat needed to raise the temperature of 1 g of pure water 1 oC. Used except when referring to food a Calorie, written with a capital C, always refers to the energy in food 1 Calorie = 1 kilocalorie = 1000 cal. ...
... heat needed to raise the temperature of 1 g of pure water 1 oC. Used except when referring to food a Calorie, written with a capital C, always refers to the energy in food 1 Calorie = 1 kilocalorie = 1000 cal. ...
PPT - Weizmann Institute of Science
... Light atoms and molecules with energies from 300 eV to 25 keV are scattered under a grazing angle of incidence from a LiF(001) surface…. Experimental results for scattering of H, D, 3He, and 4He atoms as well as H2 and D2 molecules can be unequivocally referred to atom diffraction with de Broglie wa ...
... Light atoms and molecules with energies from 300 eV to 25 keV are scattered under a grazing angle of incidence from a LiF(001) surface…. Experimental results for scattering of H, D, 3He, and 4He atoms as well as H2 and D2 molecules can be unequivocally referred to atom diffraction with de Broglie wa ...
Thermochemistry
... • The study of energy and its transformations is known as THERMODYNAMICS. • It studies the relationships between heat, work and the energy content of a system. • In this chapter we will examine an aspect of thermodynamics that involves the relationships between chemical reactions and energy changes ...
... • The study of energy and its transformations is known as THERMODYNAMICS. • It studies the relationships between heat, work and the energy content of a system. • In this chapter we will examine an aspect of thermodynamics that involves the relationships between chemical reactions and energy changes ...
Thermochemistry
... How does potential energy differ from kinetic energy? How is potential energy converted to kinetic energy? ...
... How does potential energy differ from kinetic energy? How is potential energy converted to kinetic energy? ...
Thermodynamics
... thermodynamical systems at near-equilibrium, using macroscopic, empirical properties directly measurable in the laboratory. It is used to model exchanges of energy, work and heat based on the laws of thermodynamics. Statistical mechanics (or statistical thermodynamics) gives thermodynamics a molec ...
... thermodynamical systems at near-equilibrium, using macroscopic, empirical properties directly measurable in the laboratory. It is used to model exchanges of energy, work and heat based on the laws of thermodynamics. Statistical mechanics (or statistical thermodynamics) gives thermodynamics a molec ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.