Announcements
... 3 For each force acting on one object, an equal magnitude and opposite direction force acts on another object. l Know implications of Newton’s 3 laws, for example, the Moon pulls as hard on the Earth as the Earth pulls on the Moon (3rd law) l Know how to carry out simple problems involving Newt ...
... 3 For each force acting on one object, an equal magnitude and opposite direction force acts on another object. l Know implications of Newton’s 3 laws, for example, the Moon pulls as hard on the Earth as the Earth pulls on the Moon (3rd law) l Know how to carry out simple problems involving Newt ...
y 1
... If the spring is compressed an amount A and the block released from rest, how high will it go from its initial position? ...
... If the spring is compressed an amount A and the block released from rest, how high will it go from its initial position? ...
Set 3
... If the work done on a system is reverseable, we call it configuration work. This is because in almost all cases, reverseable processes do something to change the macroscopic configuration of the system. This in general can be undone. Consider the compression of an ideal gas by a piston. Of the syste ...
... If the work done on a system is reverseable, we call it configuration work. This is because in almost all cases, reverseable processes do something to change the macroscopic configuration of the system. This in general can be undone. Consider the compression of an ideal gas by a piston. Of the syste ...
Standard - Peak to Peak Charter School
... Define, classify and describe basic forms of energy such as kinetic (mechanical) energy, gravitational potential energy, and work, heat energy, wind energy, atomic and nuclear energy, and geothermal energy WEM.3. Use the work - kinetic energy theorem to solve dynamics problems WEM.4. Explain the phy ...
... Define, classify and describe basic forms of energy such as kinetic (mechanical) energy, gravitational potential energy, and work, heat energy, wind energy, atomic and nuclear energy, and geothermal energy WEM.3. Use the work - kinetic energy theorem to solve dynamics problems WEM.4. Explain the phy ...
Lecture 19: Building Atoms and Molecules
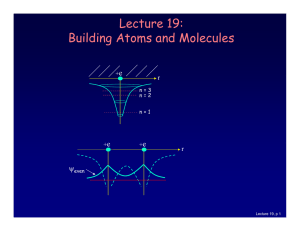
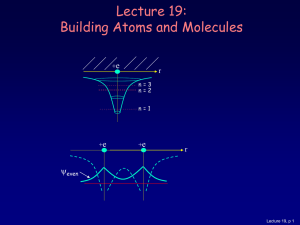
... For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) 4 What energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that eac ...
... For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) 4 What energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that eac ...
Structure of atoms and solids
... The high electrical and thermal conductivities of metals follows from the ability of these free electrons to freely move throughout their crystal structure. This is not the case in covalent or ionic bonding where electrons are tightly bound to single or groups of atoms. Unlike other crystals, metals ...
... The high electrical and thermal conductivities of metals follows from the ability of these free electrons to freely move throughout their crystal structure. This is not the case in covalent or ionic bonding where electrons are tightly bound to single or groups of atoms. Unlike other crystals, metals ...
Chapter 2. The First Law
... 2.1 Work, heat, and energy (b) Molecular interpretation 1. Heating is the transfer of energy that makes use of disorderly molecular motion (thermal motion) in the surroundings 2. Work is the transfer of energy that makes use of organized motion in the surrounding 3. The distinction between work ...
... 2.1 Work, heat, and energy (b) Molecular interpretation 1. Heating is the transfer of energy that makes use of disorderly molecular motion (thermal motion) in the surroundings 2. Work is the transfer of energy that makes use of organized motion in the surrounding 3. The distinction between work ...
Conservation of Energy
... warmed, it becomes buoyant and begins to rise, acquiring kinetic energy. Also, heating affects the kinetic energy of molecules at a microscopic level. Molecules will vibrate more vigorously as they are heated. We have a term called thermodynamic energy which wraps up this internal energy (vibrating ...
... warmed, it becomes buoyant and begins to rise, acquiring kinetic energy. Also, heating affects the kinetic energy of molecules at a microscopic level. Molecules will vibrate more vigorously as they are heated. We have a term called thermodynamic energy which wraps up this internal energy (vibrating ...
pptx
... DFT is a ground-state theory for electrons But many processes involve exciting electrons: • Transport of electrons, electron energy levels • Excited electrons ...
... DFT is a ground-state theory for electrons But many processes involve exciting electrons: • Transport of electrons, electron energy levels • Excited electrons ...
Significant decrease of the lattice thermal conductivity due to phonon
... SIGNIFICANT DECREASE OF THE LATTICE THERMAL . . . ...
... SIGNIFICANT DECREASE OF THE LATTICE THERMAL . . . ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.