Final Review
... 50. __________________ is stored energy from position. You have it if you stand above ground level or stretch a spring. It’s a type of mechanical energy. 51. The energy released from the breaking of chemical bonds through chemical reactions is known as __________________. Food, gasoline, and batter ...
... 50. __________________ is stored energy from position. You have it if you stand above ground level or stretch a spring. It’s a type of mechanical energy. 51. The energy released from the breaking of chemical bonds through chemical reactions is known as __________________. Food, gasoline, and batter ...
Name - Net Start Class
... All objects are made of particles in constant random motion. As these particles interact, their kinetic and potential energies may change, but the sum of kinetic energy and potential energy does not change. This total energy is called the internal energy of the object. An object’s Internal Energy de ...
... All objects are made of particles in constant random motion. As these particles interact, their kinetic and potential energies may change, but the sum of kinetic energy and potential energy does not change. This total energy is called the internal energy of the object. An object’s Internal Energy de ...
Rotational Motion
... The kinetic energy in a fluid is the same as for any other mass: K = ½ mv2. The change in potential energy is: U = mgh. The work done on a fluid is due to pressure. • Pressure acting on a volume: W = PAx = PV. ...
... The kinetic energy in a fluid is the same as for any other mass: K = ½ mv2. The change in potential energy is: U = mgh. The work done on a fluid is due to pressure. • Pressure acting on a volume: W = PAx = PV. ...
5,6 Quiz - mvhs
... Use this value of ΔHrxn and other data from thermodynamic quantities table to calculate the standard enthalpy (heat) of formation of cyclopropane. 3. Calculate the frequency (Hz), wavelength (nm) and energy/mol of the emitted photon when an electron drops from the n=4 to the n=2 level in a hydrogen ...
... Use this value of ΔHrxn and other data from thermodynamic quantities table to calculate the standard enthalpy (heat) of formation of cyclopropane. 3. Calculate the frequency (Hz), wavelength (nm) and energy/mol of the emitted photon when an electron drops from the n=4 to the n=2 level in a hydrogen ...
Cold encounters: Electrons and molecules
... collision energy, in atomic units. This rate of change may be obtained by suitable fitting of the data in figure 2, resulting in a lifetime of CO 2- at 10 meV impact energy of 8.48±0.lxlO- IS seconds. But how can this be? An s-wave is attempting to attach to CO 2• The LUMO of CO 2 is however of p-li ...
... collision energy, in atomic units. This rate of change may be obtained by suitable fitting of the data in figure 2, resulting in a lifetime of CO 2- at 10 meV impact energy of 8.48±0.lxlO- IS seconds. But how can this be? An s-wave is attempting to attach to CO 2• The LUMO of CO 2 is however of p-li ...
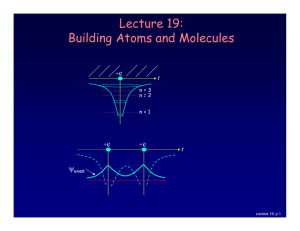
Lecture 19: Building Atoms and Molecules
... For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) 4 What energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that eac ...
... For atoms with many electrons (e.g., carbon: 6, iron: 26, etc.) 4 What energies do the electrons have? “Pauli Exclusion Principle” (1925) No two electrons can be in the same quantum state. For example, in a given atom they cannot have the same set of quantum numbers n, l, ml, ms. This means that eac ...
7th grade HA Knowledge Map 2013
... 110. Energy is the ability to do work, and can be classified into two general types: kinetic and potential energy. 111. Kinetic energy is the energy of motion and depends on the objects mass and speed. 112. The formula for kinetic energy is KE = (mv2 ) ÷ 2 where m stands for the mass of the object i ...
... 110. Energy is the ability to do work, and can be classified into two general types: kinetic and potential energy. 111. Kinetic energy is the energy of motion and depends on the objects mass and speed. 112. The formula for kinetic energy is KE = (mv2 ) ÷ 2 where m stands for the mass of the object i ...
FIRST LAW OF THERMODYNAMICS
... first law of thermodynamics can be applied to a system to evaluate the changes in its energy when it undergoes a change of state while interacting with its surroundings. The processes that are usually encountered in thermodynamic analysis of systems can be identified as any one or a combination of t ...
... first law of thermodynamics can be applied to a system to evaluate the changes in its energy when it undergoes a change of state while interacting with its surroundings. The processes that are usually encountered in thermodynamic analysis of systems can be identified as any one or a combination of t ...
Lecture 5: Heat transmission
... the radiator below. In this way warm air moves to the other side of the room. Once on the other side of the room the air drops down both because it has cooled a little and because the air behind it continues to push on it. The air then continues to circulate back to the radiator and repeat the proce ...
... the radiator below. In this way warm air moves to the other side of the room. Once on the other side of the room the air drops down both because it has cooled a little and because the air behind it continues to push on it. The air then continues to circulate back to the radiator and repeat the proce ...
Anonymous-IntroductiontoThermodynamics-qsp_chapte+
... • We can allow heat Q to enter the system. The first statement is fundamental since it asserts that, by doing work on the system alone, we can raise the internal energy to any given value, quite independent of what we might mean by heat. This is the clue to how we define what heat is. We write down ...
... • We can allow heat Q to enter the system. The first statement is fundamental since it asserts that, by doing work on the system alone, we can raise the internal energy to any given value, quite independent of what we might mean by heat. This is the clue to how we define what heat is. We write down ...
2 Chemical bonding is a genuinely quantum effect, which cannot be
... between two nuclei? In many cases, we do not have a large electron delocalization, thus there is nothing like a sea of electrons (which is the case in a metal). In organic molecules, we have two electrons in every bonding orbital, and that is how covalent bonding is explained. The main idea behind t ...
... between two nuclei? In many cases, we do not have a large electron delocalization, thus there is nothing like a sea of electrons (which is the case in a metal). In organic molecules, we have two electrons in every bonding orbital, and that is how covalent bonding is explained. The main idea behind t ...
Second Law of thermodynamics
... • Ideal- the process is reversible, that is, is done so slow that can be considered a series of equilibrium states, so the whole process can be done in reverse with no change in the magnitude of work done or heat exchanged. • Real- the process is done more quickly so there is heat lost because of fr ...
... • Ideal- the process is reversible, that is, is done so slow that can be considered a series of equilibrium states, so the whole process can be done in reverse with no change in the magnitude of work done or heat exchanged. • Real- the process is done more quickly so there is heat lost because of fr ...
Quiz 2_Fulltime
... A cart at the top of a 0.3 Km hill has a mass of 500 g? Assuming acceleration due to gravity to be 10m s/s. ...
... A cart at the top of a 0.3 Km hill has a mass of 500 g? Assuming acceleration due to gravity to be 10m s/s. ...
Biogeochemical cycles and thermodynamics
... Gibbs free energy is an extensive state function meaning that the value of G depends on the state and mass of the system under consideration. It is a function of several parameters, including internal energy (U), enthalpy (H), entropy (S) and temperature (T) G = H – TS or G = H - TS Internal ener ...
... Gibbs free energy is an extensive state function meaning that the value of G depends on the state and mass of the system under consideration. It is a function of several parameters, including internal energy (U), enthalpy (H), entropy (S) and temperature (T) G = H – TS or G = H - TS Internal ener ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.