* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Second Law of thermodynamics
Heat capacity wikipedia , lookup
Thermoregulation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Heat equation wikipedia , lookup
Calorimetry wikipedia , lookup
Temperature wikipedia , lookup
Conservation of energy wikipedia , lookup
Maximum entropy thermodynamics wikipedia , lookup
Heat transfer wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
Thermal conduction wikipedia , lookup
Heat transfer physics wikipedia , lookup
Entropy in thermodynamics and information theory wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Extremal principles in non-equilibrium thermodynamics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermodynamic system wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
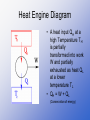
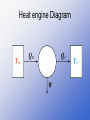
THERMODYNAMICS CH 15 THERMODYNAMICS • Thermodynamics is the study of processes in which energy is transferred as heat and as work • System is any object or set of objects that we wish to consider • Environment is everything else in the universe OPEN AND CLOSED SYSTEMS • Closed system is one for which no mass enters or leaves (but energy may be exchanged with the environment) Ex: idealized systems studied in physics • Open system is one for which mass may enter or leave (as well as energy) Ex: plants and animals Isolated System • Isolated system is a closed system where no energy in any form passes across its boundaries 1ST Law of Thermodynamics • The change in internal energy of a closed system, ∆U, will be equal to the sum of the energy transferred across the system boundary by heat (Q) and the energy transferred by work (W) • ∆U = Q + W Sign Convention • Energy of any kind that goes into the system is + • Energy of any kind that comes out of the system is – First Law of Thermodynamics is conservation of energy • It is one of the great laws of physics • Its validity rests on experiments (such as Joule’s) in which no exceptions have been seen • Internal energy is the sum total of all the energy the molecules of the system. It is a property of a system like pressure, volume and temperature • Work and heat are not properties of a system First Law of Thermodynamics applied to some simple systems • Isothermal Process is an idealized process that is carried out at constant temperature (∆T = 0) • An ideal gas in a cylinder fitted with a movable piston PV diagram for an ideal gas undergoing isothermal processes • If the temperature is to remain constant, the gas must expand and do an amount of work W on the environment (it exerts a force on the piston and moves it through a distance) • ∆U = 3 N k∆T = 0 (since the • 2 temperature is kept constant) U=Q+W=0 W = -Q (the work done by the gas in an isothermal process equals the heat lost to the environment) Adiabatic Process • Adiabatic process is one in which no heat is allowed to flow into or out of the system: Q=0 • It can occur if the system is extremely well insulated, or the process happens so quickly that the heat-which flows slowlyhas no time to flow in or out. PV diagram for adiabatic process • Since Q = 0, ∆U = W (The internal energy decreases if the gas expands) • The temperature decreases as well since ∆U = 3 N k ∆T 2 • In an adiabatic compression work is done on the system so U and T increases Isobaric Process • Isobaric process is one in which the pressure is kept constant, so the process is represented by a straight line on the PV diagram Isochoric Process • Isochoric or isovolumetric process is one which the volume does not change PV diagram for Different processes Work done in volume changes • W = F d = Pad • W = - P ∆V • Work done by a gas is equal to the area under the PV curve The Second Law of Thermodynamics • The First Law of Thermodynamics states that energy is conserved • Some processes in nature do not occur in reverse even though they wouldn’t violate the First Law Ex: broken glass back to be together spontaneously Essential Question • Which processes occur in nature and which do not? • On the second half of xix century scientists came to formulate a new principle known as the second law of thermodynamics • It is a statement about which processes occur in nature and which do not The second law of thermodynamics • It is stated in a variety of ways • A general statement is based on the study of heat engines Heat Engines • A heat engine is any device that changes thermal energy into mechanical work Ex: steam engines ( most electricity today is generated with steam turbines) Car engines (internal combustion engine) Heat Engine Diagram • A heat input QH at a high Temperature TH is partially transformed into work W and partially exhausted as heat QL at a lower temperature TL • QH = W + QL (Conservation of energy) Diagram of Reciprocating type of steam engine Diagram of Turbine steam Engine Internal Combustion Engine • In an internal combustion engine, the high temperature is achieved by burning the gasolineair mixture in the cylinder itself (ignited by the spark plug) Why a ∆T is needed to run a heat engine? • Same temperature would mean that the pressure of the gas being exhausted would be the same as that on intake. • Work would be done by the gas on the piston when it expanded but the same amount of work would be done by the piston to force the gas to exhaust. No net work! Efficiency of a Heat Engine • A net amount of work is obtained only if there is a difference in temperature. • QH = W + QL (Conservation of energy) • The efficiency, e, of any heat engine can be defined as the ratio of the work it does, W, to the heat input, QH e = W = QH – QL = 1 - QL QH QH QH Carnot Engines • It is an ideal engine, investigated by the French scientist Sadi Carnot (1796-1832) to see how to increase the efficiency of a real engine • No Carnot engine actually exists, but as a theoretical engine it played an important role in the development of thermodynamics PV Diagram of Carnot cycle • 1-2: Isothermal expansion • 2-3: Adiabatic expansion • 3-4: Isothermal compression • 4-1: Adiabatic compression Ideal x Real Process • Ideal- the process is reversible, that is, is done so slow that can be considered a series of equilibrium states, so the whole process can be done in reverse with no change in the magnitude of work done or heat exchanged. • Real- the process is done more quickly so there is heat lost because of friction and turbulence, so the process cannot be done precisely in reverse, the process is then called irreversible. Carnot efficiency • For ideal engines • Q ˜T (T in kelvin) • eideal=TH – TL= 1-TL TH TH Real engines that are well designed reach 60 to 80 percent of the Carnot efficiency Entropy • It was not until the latter half of the nineteenth century the second law of thermodynamics was finely stated in a general way in terms of a quantity called entropy. • Entropy is a measure of order or disorder of a system • Entropy is a function of state of a system • Like potential energy, it is the change in entropy during a process that is important not the absolute amount Entropy • The change in entropy ∆S of a system when an amount of heat Q is added to it by a reversible process at constant temperature: • ∆S = Q T (T in Kelvin) Entropy • The entropy of an isolated system never decreases. It can only stay the same (ideal, reversible processes) or increase (real processes) • ∆S > 0 • If the system is not isolated: • ∆S = ∆SS + ∆Senv ≥ 0 Second Law of thermodynamics (General Statement) • The total entropy of any system plus that of its environment increases as a result of any natural process • Or • Natural processes tend to move toward a state of greater disorder Order to Disorder • The second law introduces a new quantity, S, that is not conserved in natural processes, it always increase in time. • Entropy concept is very abstract. To get a better feel of it, we can relate it to order and disorder • Entropy of a system is a measure of the disorder of the system Which process occur in nature and which not? • The normal course of events is an increase of disorder (entropy) • Ex: a solid coffee cup is a more “orderly” object than the pieces of a broken cup Cups break when they fall, but they do not spontaneously mend themselves Thermodynamocs Laws • 0th: A equilibrium B » A equilibrium C B equilibrium C • 1st: Conservation of energy: ∆U = Q – W • 2nd: Which processes occur in nature and which do not (Entropy). Natural processes tend to move toward a state of greater disorder (∆S ≥ 0) • 3rd: From careful experimentation absolute zero is unattainable. Diagram of energy transfer for a heat pump • The operating principle of refrigerators, air conditioners, and heat pumps is just the reverse of a heat engine. • Each operates to transfer heat out of a cool environment into a warm environment Heat engine Diagram Heat Pump • Refrigerator • Heat pump or air conditioner