* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download HOMEWORK #2
Dynamic insulation wikipedia , lookup
Solar water heating wikipedia , lookup
Building insulation materials wikipedia , lookup
Heat exchanger wikipedia , lookup
Solar air conditioning wikipedia , lookup
Heat equation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Intercooler wikipedia , lookup
Thermal conduction wikipedia , lookup
Cogeneration wikipedia , lookup
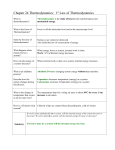
HOMEWORK #2 Name: _______________________ Date: ___________ Period: _____ Heat Engine and Entropy THE LAWS OF THERMODYNAMICS 1st: Energy can be transformed (changed from one form to another), but it can neither be created nor destroyed. 2nd: In a system, a process that occurs will tend to increase the total entropy of the universe. (Heat will not of itself flow from cold to hot.) REVIEW QUESTIONS – check concepts 1. What is the meaning of the Greek words from which we get the word thermodynamics? 2. What is the lowest possible temperature on the Celsius scale? On the Kelvin scale? (PHYZR#12) (PHYZR#12) 3. How does the law of conservation of energy relate to the first law of thermodynamics? 4. What is the relationship between heat flow input, work done, and heat flow output? (Page#4) (Page#4) 5. What is the physicist’s term for a measure of disorder (messiness)? (PHYZR#14) 6. With respect to orderly and disorderly states, what do natural system tend to do? (PHYZR#14) 7. Can a disorderly state ever transform to an orderly state? Explain. (Page#6) 8. Is a melting ice cube an example of increasing order or decreasing order? (Page#6) HEAT ENGINE PROBLEMS 9. Use conservation of energy to determine the missing energy. 20 J 50 J 200 J 10 J 200 J 80 J 900 J 700 J Fremont Physics ©Kepple 2012 40 J 100 J 600 J 120 J 70 J 20 J 34 J 12 J Thermodynamics – HW-B 1/11/13 PLUG AND CHUG – using equations 10. An engine absorbs 300 J of heat and exhausts 200 J. How much work is done? 11. A heat engine does 50 J of work with 250 J of exhaust. How much heat was absorbed? 12. How much heat is ejected each cycle by a heat engine that does 60 J of work in each cycle and absorbs 90 J of heat in each cycle? 13. If a heat engine does 100 J of work in each cycle and ejects 125 J in each cycle, how much heat has to be added in each cycle? THINK AND EXPLAIN – critical thinking 14. Pretend you place a cup of hot coffee in your refridgerator and the coffee absorbs 10 J of energy from the refridgerator and becomes warmer, while the refridgerator gives up 10 J of energy and becomes colder. Would this energy transfer violate the first law of thermodynamics? Would this energy transfer violate the second law of thermodynamics? Explain. 15. Water put into the freezer compartment in your refrigerator goes to a state of less molecular disorder when it freezes. Is this an exception to the entropy principle? Explain. 16. What is the connection between entropy and time? Explain how you can tell the difference between the past and the future by using entropy.