Modern Thermodynamics
... 14.10 Appendix: The Cyclic Triple Derivative Rule . . . . . . . . . . . . . . . . . ...
... 14.10 Appendix: The Cyclic Triple Derivative Rule . . . . . . . . . . . . . . . . . ...
2007 exam 3 with answers
... is deposited into equal masses of the two materials. What is true about the change in temperature that results? 1. The temperature change for Cu will be 10-fold higher than that for water. correct 2. The temperature change for water will be 10-fold higher than that for Cu. 3. The temperature changes ...
... is deposited into equal masses of the two materials. What is true about the change in temperature that results? 1. The temperature change for Cu will be 10-fold higher than that for water. correct 2. The temperature change for water will be 10-fold higher than that for Cu. 3. The temperature changes ...
Thermodynamics By S K Mondal
... 12. Two blocks which are at different states are brought into contact with each other and allowed to reach a final state of thermal equilibrium. The final temperature attained is specified by the (a) Zeroth law of thermodynamics (b) First law of thermodynamics [IES-1998] (c) Second law of thermodyn ...
... 12. Two blocks which are at different states are brought into contact with each other and allowed to reach a final state of thermal equilibrium. The final temperature attained is specified by the (a) Zeroth law of thermodynamics (b) First law of thermodynamics [IES-1998] (c) Second law of thermodyn ...
Module P7.4 Specific heat, latent heat and entropy
... pressure, ☞temperature, volume and work. It would also be helpful if you have some understanding of the following terms equation of state (of an ideal gas), first law of thermodynamics, function of state, heat, ideal gas, internal energy, quasistatic process and thermal equilibrium. The terms in thi ...
... pressure, ☞temperature, volume and work. It would also be helpful if you have some understanding of the following terms equation of state (of an ideal gas), first law of thermodynamics, function of state, heat, ideal gas, internal energy, quasistatic process and thermal equilibrium. The terms in thi ...
Chemical Thermodynamics
... Suppose, for example, that the system under study is a mass of steam heated by the combustion of several hundred pounds of coal and enclosed within a cylinder housing a piston attached to the crankshaft of a large steam engine. The gas is not ideal, and the cylinder is not frictionless. Nonetheless, ...
... Suppose, for example, that the system under study is a mass of steam heated by the combustion of several hundred pounds of coal and enclosed within a cylinder housing a piston attached to the crankshaft of a large steam engine. The gas is not ideal, and the cylinder is not frictionless. Nonetheless, ...
Boundless Study Slides
... • the first law of thermodynamics A version of the law of conservation of energy, specialized for thermodynamical systems. Usually expressed as ΔU=Q−W. • the second law of thermodynamics A law stating that states that the entropy of an isolated system never decreases, because isolated systems sponta ...
... • the first law of thermodynamics A version of the law of conservation of energy, specialized for thermodynamical systems. Usually expressed as ΔU=Q−W. • the second law of thermodynamics A law stating that states that the entropy of an isolated system never decreases, because isolated systems sponta ...
Enthalpy, Entropy, Mollier Diagram and Steam
... absolute entropies that have occurred over the life of the system. stotal = ∑ ∆si ...
... absolute entropies that have occurred over the life of the system. stotal = ∑ ∆si ...
Chapter 19 Thermodynamics - Farmingdale State College
... Thus, equation 19.11 represents the net work done by the gas in this particular cyclic process. Note that pA _ pD is one side of the rectangular path of figure 19.2(a) while VB _ VA is the other side of that rectangle. Hence, their product in equation 19.11 represents the entire area of the rectangl ...
... Thus, equation 19.11 represents the net work done by the gas in this particular cyclic process. Note that pA _ pD is one side of the rectangular path of figure 19.2(a) while VB _ VA is the other side of that rectangle. Hence, their product in equation 19.11 represents the entire area of the rectangl ...
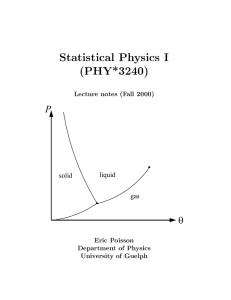
book - University of Guelph Physics
... The existence of the relation g(P, V ) = θ for isotherms, inferred empirically above, can also be justified by rigourous mathematics. We will now go through this argument. This will serve to illustrate a major theme of thermodynamics: Simple physical ideas (such as the zeroth law) can go very far wh ...
... The existence of the relation g(P, V ) = θ for isotherms, inferred empirically above, can also be justified by rigourous mathematics. We will now go through this argument. This will serve to illustrate a major theme of thermodynamics: Simple physical ideas (such as the zeroth law) can go very far wh ...
Chapter 2
... To define temperature more carefully, consider two systems separated by an insulating wall.3 A wall is said to be insulating if there is no energy transfer through the wall due to temperature differences between regions on both sides of the wall. If the wall between the two systems were conducting, ...
... To define temperature more carefully, consider two systems separated by an insulating wall.3 A wall is said to be insulating if there is no energy transfer through the wall due to temperature differences between regions on both sides of the wall. If the wall between the two systems were conducting, ...
Equilibrium Statistical Mechanics
... are separately at equilibrium: if we lift one (or more) constraint the final entropy, after the re-establisment of equilibrium, must be greater or equal to the initial i are such entropy. The new values of Em , Vm , Nm that the entropy is increased or remains the same. In summary: the entropy of an ...
... are separately at equilibrium: if we lift one (or more) constraint the final entropy, after the re-establisment of equilibrium, must be greater or equal to the initial i are such entropy. The new values of Em , Vm , Nm that the entropy is increased or remains the same. In summary: the entropy of an ...
Thermodynamics
... Clausius, and William Thomson (Lord Kelvin). The foundations of statistical thermodynamics were set out by physicists such as James Clerk Maxwell, Ludwig Boltzmann, Max Planck, Rudolf Clausius and J. Willard Gibbs. During the years 1873-76 the American mathematical physicist Josiah Willard Gibbs pub ...
... Clausius, and William Thomson (Lord Kelvin). The foundations of statistical thermodynamics were set out by physicists such as James Clerk Maxwell, Ludwig Boltzmann, Max Planck, Rudolf Clausius and J. Willard Gibbs. During the years 1873-76 the American mathematical physicist Josiah Willard Gibbs pub ...
Heat Engines, Entropy, and the Second Law of Thermodynamics P
... again affected because energy is being added to the surroundings from the gas. If this energy could somehow be used to drive the engine that we have used to compress the gas, then the net energy transfer to the surroundings would be zero. In this way, the system and its surroundings could be returne ...
... again affected because energy is being added to the surroundings from the gas. If this energy could somehow be used to drive the engine that we have used to compress the gas, then the net energy transfer to the surroundings would be zero. In this way, the system and its surroundings could be returne ...
Entropy generation minimization of one and two
... The control volumes CVref,1..N surround the refrigerant flow in each of the circuits. These calculate the average heat transfer coefficient and the pressure drop of the refrigerant. CVhyd,1..N surrounds the hydrogen flow of each of the circuits and calculates the average heat transfer coefficient an ...
... The control volumes CVref,1..N surround the refrigerant flow in each of the circuits. These calculate the average heat transfer coefficient and the pressure drop of the refrigerant. CVhyd,1..N surrounds the hydrogen flow of each of the circuits and calculates the average heat transfer coefficient an ...
Statistical Thermodynamics and Stochastic The
... and following Joule, Clausius interpreted the passage of heat as the transformation of different kinds of energy, in which the total energy is conserved. To generate work, heat must be transferred from a reservoir at a high temperature to one at a lower temperature, and Clausius here introduced the ...
... and following Joule, Clausius interpreted the passage of heat as the transformation of different kinds of energy, in which the total energy is conserved. To generate work, heat must be transferred from a reservoir at a high temperature to one at a lower temperature, and Clausius here introduced the ...
20 Entropy and the Second Law of Thermodynamics
... one-way processes irreversible, meaning that they cannot be reversed by means of only small changes in their environment. Many chemical transformations are also irreversible. For example, when methane gas is burned, each methane molecule mixes with an oxygen molecule. Water vapor and carbon dioxide ...
... one-way processes irreversible, meaning that they cannot be reversed by means of only small changes in their environment. Many chemical transformations are also irreversible. For example, when methane gas is burned, each methane molecule mixes with an oxygen molecule. Water vapor and carbon dioxide ...
Thermodynamics Of Chemical Processes
... Thermodynamics is one of the sciences on which Chemical Engineering is based upon. There are many definitions of the science of thermodynamics. The word originates from the Greek language. The Greek words thermo and dynamics mean heat and motion respectively. Thus, thermodynamics can be explained as ...
... Thermodynamics is one of the sciences on which Chemical Engineering is based upon. There are many definitions of the science of thermodynamics. The word originates from the Greek language. The Greek words thermo and dynamics mean heat and motion respectively. Thus, thermodynamics can be explained as ...
thermodynamics type 1
... of quantities of heat & work. It may be defined as the branch of science which deals with energy changes associated with various physical & chemical processes. The entire formulation of thermodynamics is based on a few (Three) fundamental laws which have been established on the basis of human experi ...
... of quantities of heat & work. It may be defined as the branch of science which deals with energy changes associated with various physical & chemical processes. The entire formulation of thermodynamics is based on a few (Three) fundamental laws which have been established on the basis of human experi ...