Chapter 5 auxiliary functions
... * From the second law of thermodynamics : q ≤ T(S2 –S1) ≤ – ΔGَw therefore for reversible processes that occur at constant temperature and pressure ; the maximum amount of work , other than the p – v work is given by equation : max = – ΔGَw * again the pervious inequality can b written as ; = – (ΔG ...
... * From the second law of thermodynamics : q ≤ T(S2 –S1) ≤ – ΔGَw therefore for reversible processes that occur at constant temperature and pressure ; the maximum amount of work , other than the p – v work is given by equation : max = – ΔGَw * again the pervious inequality can b written as ; = – (ΔG ...
12. THE LAWS OF THERMODYNAMICS Key Words
... process, some of the heat can be transformed into mechanical work. Equation (12-9) expresses the fundamental upper limit to the efficiency. No engine operating between the same two temperatures can be more efficient than a Carnot engine. Real engines always have efficiency lower than this because of ...
... process, some of the heat can be transformed into mechanical work. Equation (12-9) expresses the fundamental upper limit to the efficiency. No engine operating between the same two temperatures can be more efficient than a Carnot engine. Real engines always have efficiency lower than this because of ...
Second Law of Thermodynamics
... For the gas to do positive work, the cycle must be traversed in a clockwise manner. Positive heat is added to the gas as it proceeds from state C to state D. The net work done by the gas is proportional to the area inside the closed curve. The heat transferred as the gas proceeds from state B to sta ...
... For the gas to do positive work, the cycle must be traversed in a clockwise manner. Positive heat is added to the gas as it proceeds from state C to state D. The net work done by the gas is proportional to the area inside the closed curve. The heat transferred as the gas proceeds from state B to sta ...
Chapter 19 Chemical Thermodynamics
... • Entropy can be thought of as a measure of the randomness of a system. • It is related to the various modes of motion in molecules. ...
... • Entropy can be thought of as a measure of the randomness of a system. • It is related to the various modes of motion in molecules. ...
Chapter 19 Chemical Thermodynamics
... • Entropy can be thought of as a measure of the randomness of a system. • It is related to the various modes of motion in molecules. ...
... • Entropy can be thought of as a measure of the randomness of a system. • It is related to the various modes of motion in molecules. ...
Lecture 4: 09.16.05 Temperature, heat, and entropy
... The Third Law and calculation of absolute entropies •� The third law, like the other laws of thermodynamics, is derived from empirical observations made by scientists studying the behavior of thermodynamic systems. The third law derived from experiments looking at the behavior of heat capacities and ...
... The Third Law and calculation of absolute entropies •� The third law, like the other laws of thermodynamics, is derived from empirical observations made by scientists studying the behavior of thermodynamic systems. The third law derived from experiments looking at the behavior of heat capacities and ...
unit ii chemical thermodynamics
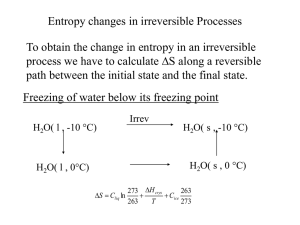
... Second law Entropy - entropy change for an ideal gas, reversible and irreversible processes ,entropy of phase transitions. Clausius inequality. Free energy and work function: Helmholtz and Gibbs free energy functions Criteria of spontaneity Gibbs-Helmholtz equation Clausius-Clapeyron ...
... Second law Entropy - entropy change for an ideal gas, reversible and irreversible processes ,entropy of phase transitions. Clausius inequality. Free energy and work function: Helmholtz and Gibbs free energy functions Criteria of spontaneity Gibbs-Helmholtz equation Clausius-Clapeyron ...
ONSAGER`S VARIATIONAL PRINCIPLE AND ITS APPLICATIONS
... T and V , then S and p are readily calculated from its partial derivatives. On the Helmholtz free energy F : While the definition of F ≡ U − T S is applicable to arbitrary macroscopic states (so long as there is partial equilibrium and hence the entropy can be defined), the total differential dF = −SdT ...
... T and V , then S and p are readily calculated from its partial derivatives. On the Helmholtz free energy F : While the definition of F ≡ U − T S is applicable to arbitrary macroscopic states (so long as there is partial equilibrium and hence the entropy can be defined), the total differential dF = −SdT ...
heat engine
... Consider a system taken through an arbitrary (non-Carnot) reversible cycle Entropy S is a state function S depends only on the properties of a given equilibrium state S = 0 ...
... Consider a system taken through an arbitrary (non-Carnot) reversible cycle Entropy S is a state function S depends only on the properties of a given equilibrium state S = 0 ...
16 3.0 Chapter Contents 3.1 The Entropy and Internal Energy
... where A is a scaling parameter. Differentiating with respect to A and then letting A = 1 we obtain the energy function in the form ...
... where A is a scaling parameter. Differentiating with respect to A and then letting A = 1 we obtain the energy function in the form ...
Lecture VIII_IX
... temperature and expansion L is given by f(T,L) = aT(L-L0) where a and L0 are constants. • (a)Use Maxwell relations to determine the entropy and enthalpy at constant T and p. • (b) If you adiabatically stretch a rubber band by small amount, its temperature increases but volume does not change. Derive ...
... temperature and expansion L is given by f(T,L) = aT(L-L0) where a and L0 are constants. • (a)Use Maxwell relations to determine the entropy and enthalpy at constant T and p. • (b) If you adiabatically stretch a rubber band by small amount, its temperature increases but volume does not change. Derive ...
More Thermodynamics
... ► Net Heat Absorbed: QH – QC ► Net Change in U is 0 (initial = final) ► W = QH – QC so heat is converted to work! QH energy input QC is exhaust energy ► Efficiency is ...
... ► Net Heat Absorbed: QH – QC ► Net Change in U is 0 (initial = final) ► W = QH – QC so heat is converted to work! QH energy input QC is exhaust energy ► Efficiency is ...