Document
... arrangements that give state C. The macrostate with the highest entropy also has the greatest dispersal of energy. Therefore, state C has higher entropy than either state A or state B. There is six times the probability of having the state C macrostate than either state A or state B. © 2014 Pearson ...
... arrangements that give state C. The macrostate with the highest entropy also has the greatest dispersal of energy. Therefore, state C has higher entropy than either state A or state B. There is six times the probability of having the state C macrostate than either state A or state B. © 2014 Pearson ...
Basic Thermodynamics - CERN Accelerator School
... driving forces) within the system. A system that is in thermodynamic equilibrium experiences no change when it is isolated from its surroundings. It should be stressed that thermodynamic equilibrium implies steady state, but that steady state does not always induce thermodynamic equilibrium (e.g. st ...
... driving forces) within the system. A system that is in thermodynamic equilibrium experiences no change when it is isolated from its surroundings. It should be stressed that thermodynamic equilibrium implies steady state, but that steady state does not always induce thermodynamic equilibrium (e.g. st ...
Solutions Exercises Lecture 4
... adiabatically from 2 to 10 bar. Further, it is given that for this irreversible process work needs to be done on the system. The energy required for this work is 1.4 times the energy required for the reversible process from the same initial to the same end state. With the help of this information th ...
... adiabatically from 2 to 10 bar. Further, it is given that for this irreversible process work needs to be done on the system. The energy required for this work is 1.4 times the energy required for the reversible process from the same initial to the same end state. With the help of this information th ...
Course Home - Haldia Institute of Technology
... ME201.3 Ability to understand work and heat transfer, enthalpy, entropy etc. for calculating different engineering problems. ME201.4 Ability to understand fluid statics, dynamics and kinematics and hence to gather knowledge of compressible , incompressible liquid, also knowledge of manometer, ventur ...
... ME201.3 Ability to understand work and heat transfer, enthalpy, entropy etc. for calculating different engineering problems. ME201.4 Ability to understand fluid statics, dynamics and kinematics and hence to gather knowledge of compressible , incompressible liquid, also knowledge of manometer, ventur ...
Thermodynamics
... Statistical physics, to the contrary, uses the microscopic approach to calculate macroscopic quantities that thermodynamics has to take from the experiment. The microscopic approach of the statistical physics is still much less detailed than the full dynamical description based on Newton’s equations ...
... Statistical physics, to the contrary, uses the microscopic approach to calculate macroscopic quantities that thermodynamics has to take from the experiment. The microscopic approach of the statistical physics is still much less detailed than the full dynamical description based on Newton’s equations ...
q 2 - q 1
... spontaneous change decreases with the occurrence of the natural process ( a change from non equilibrium state to equilibrium state ) until equilibrium is attained at which the capacity of the system for spontaneous change will be exhausted ; thus, if the process proceeds under infinitesimally small ...
... spontaneous change decreases with the occurrence of the natural process ( a change from non equilibrium state to equilibrium state ) until equilibrium is attained at which the capacity of the system for spontaneous change will be exhausted ; thus, if the process proceeds under infinitesimally small ...
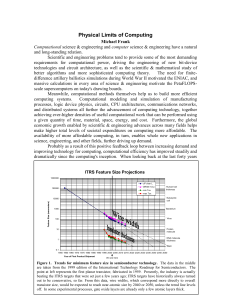
Physical Limits of Computing
... is just known entropy. Entropy is just unknown information. Interestingly, infropy is, apparently, like energy, a localized phenomenon. That is, it has a definite location in space, associated with the location of the subsystem whose state is in question. Even information about a distant object can ...
... is just known entropy. Entropy is just unknown information. Interestingly, infropy is, apparently, like energy, a localized phenomenon. That is, it has a definite location in space, associated with the location of the subsystem whose state is in question. Even information about a distant object can ...
EQATION OF STATE IN FORM WHICH RELATES MOL FRACTION
... Abstract: Most people would face a problem if there is a need to calculate the mole fraction of a substance A in a gaseous solution (a thermodynamic system containing two or more ideal gases) knowing its molarity at a given temperature and pressure. For most it would take a lot of time and calculati ...
... Abstract: Most people would face a problem if there is a need to calculate the mole fraction of a substance A in a gaseous solution (a thermodynamic system containing two or more ideal gases) knowing its molarity at a given temperature and pressure. For most it would take a lot of time and calculati ...
4. Classical Thermodynamics
... allow heat (to be defined shortly) to be transmitted between systems. If in doubt, think of a thin sheet of metal. • An isolated system, when left alone for a suitably long period of time, will relax to a state where no further change is noticeable. This state is called equilibrium Since we care not ...
... allow heat (to be defined shortly) to be transmitted between systems. If in doubt, think of a thin sheet of metal. • An isolated system, when left alone for a suitably long period of time, will relax to a state where no further change is noticeable. This state is called equilibrium Since we care not ...
heat processes
... EGM is a design concept based upon minimization of irreversible processes. It is a new philosophy: reversible processes are good, irreversible wrong. As a measure of irreversibility the rate of entropy generation in a system is considered. Entropy increase is caused by heat transfer from high to low ...
... EGM is a design concept based upon minimization of irreversible processes. It is a new philosophy: reversible processes are good, irreversible wrong. As a measure of irreversibility the rate of entropy generation in a system is considered. Entropy increase is caused by heat transfer from high to low ...
The first and second law of Thermodynamics - Ole Witt
... prerequisites for the kinetic theory of gasses. However, it would not be the case, if the molecules interacted by medium range forces. One main result from the kinetic theory of gasses is that the internal energy of an ideal gas can be expressed as. ...
... prerequisites for the kinetic theory of gasses. However, it would not be the case, if the molecules interacted by medium range forces. One main result from the kinetic theory of gasses is that the internal energy of an ideal gas can be expressed as. ...
P - School of Chemical Sciences
... http://www.scs.uiuc.edu/~makri/444-web-page/chem-444.html/444-course-planner.html ...
... http://www.scs.uiuc.edu/~makri/444-web-page/chem-444.html/444-course-planner.html ...