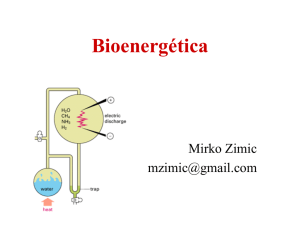
Chapter 17 - Groupfusion.net
... • The driving force for a spontaneous process is an increase in the entropy of the universe • Order (lower entropy) to disorder (higher entropy) • Thermodynamic function that describes the number of arrangements (positions and/or energy levels) available to a system in a given state. Associated with ...
... • The driving force for a spontaneous process is an increase in the entropy of the universe • Order (lower entropy) to disorder (higher entropy) • Thermodynamic function that describes the number of arrangements (positions and/or energy levels) available to a system in a given state. Associated with ...
The Second Law of Thermodynamics
... in phase with one another, as shown in Figure 4.2b. At the instant the ball leaves the floor, all the atoms in these molecules must be moving upward for proper energy transfer. It is conceivable for 2 million molecules to execute this kind of synchronized motion, but because of the magnitude of energ ...
... in phase with one another, as shown in Figure 4.2b. At the instant the ball leaves the floor, all the atoms in these molecules must be moving upward for proper energy transfer. It is conceivable for 2 million molecules to execute this kind of synchronized motion, but because of the magnitude of energ ...
Chapter Two The Thermodynamic Laws
... reservoir and produce a net amount of work." This was shown to be equivalent to the statement of Clausius. (2.3.2). Statements of the second law (2.3.2.1). Thermal reservoir Thermal reservoir, characterized by its temperature, is a reservoir of infinite heat capacity. Thermal reservoir can play the ...
... reservoir and produce a net amount of work." This was shown to be equivalent to the statement of Clausius. (2.3.2). Statements of the second law (2.3.2.1). Thermal reservoir Thermal reservoir, characterized by its temperature, is a reservoir of infinite heat capacity. Thermal reservoir can play the ...
Statistical mechanics
... Statistical mechanics or statistical thermodynamics[1] is a branch of physics that applies probability theory, which contains mathematical tools for dealing with large populations, to the study of the thermodynamic behavior of systems composed of a large number of particles. Statistical mechanics pr ...
... Statistical mechanics or statistical thermodynamics[1] is a branch of physics that applies probability theory, which contains mathematical tools for dealing with large populations, to the study of the thermodynamic behavior of systems composed of a large number of particles. Statistical mechanics pr ...
Chapter 2
... stored in sugars. Thus the internal energy of this person must have decreased by at least 4700 J. However, there are other considerations. We know that exercising raises body temperature. We shall see that this is associated with an increase in this person’s thermal energy. As long as this thermal e ...
... stored in sugars. Thus the internal energy of this person must have decreased by at least 4700 J. However, there are other considerations. We know that exercising raises body temperature. We shall see that this is associated with an increase in this person’s thermal energy. As long as this thermal e ...
Screen Version
... One way of stating the Second Law of Thermodynamics is: “only by transferring heat from a warmer to a colder body can heat can be converted into work in a cyclic process.” It can be shown that no engine can be more efficient than a reversible engine working between the same limits of temperature, a ...
... One way of stating the Second Law of Thermodynamics is: “only by transferring heat from a warmer to a colder body can heat can be converted into work in a cyclic process.” It can be shown that no engine can be more efficient than a reversible engine working between the same limits of temperature, a ...
First Law of Thermodynamics - Derry Area School District
... likely macrostate – described by p, V, and T and obeying the ideal gas law – has so many microstates associated with it that it’s the only one you have any chance of observing. • When you allow two systems at different temperatures to exchange energy with each other, the final macrostate of the syst ...
... likely macrostate – described by p, V, and T and obeying the ideal gas law – has so many microstates associated with it that it’s the only one you have any chance of observing. • When you allow two systems at different temperatures to exchange energy with each other, the final macrostate of the syst ...
slides - Biology Courses Server
... BUT, we can define the value of w (or q) for a specific process linking two states to be a change in a state function. We define the work for the reversible (infinitely slow) conversion of one state to the another, wrev , to be the change in state function ∆F . • ∆F is called the change in “free ene ...
... BUT, we can define the value of w (or q) for a specific process linking two states to be a change in a state function. We define the work for the reversible (infinitely slow) conversion of one state to the another, wrev , to be the change in state function ∆F . • ∆F is called the change in “free ene ...
Friction force: from mechanics to thermodynamics
... namics. In particular, following Stückelberg, we formulate the first and second law as time evolution described by first order differential equations. These examples will be used to illustrate the evolution of mechanical systems in the presence of friction taking into account the thermodynamical as ...
... namics. In particular, following Stückelberg, we formulate the first and second law as time evolution described by first order differential equations. These examples will be used to illustrate the evolution of mechanical systems in the presence of friction taking into account the thermodynamical as ...
THERMODYNAMICS
... The entropy of 1 mole of vapor is calculated using the entropy of 1 mole of liquid (161 J/K) to which the entropy change resulting from the heat absorption (141.9 J/K) is added: Entropy of Vapor = 161 J/K + 141.9 J/K = 303 J/K 3. Consider the combustion of propane gas: C3H8 (g) + 5 O2 (g) → 3 CO2 (g ...
... The entropy of 1 mole of vapor is calculated using the entropy of 1 mole of liquid (161 J/K) to which the entropy change resulting from the heat absorption (141.9 J/K) is added: Entropy of Vapor = 161 J/K + 141.9 J/K = 303 J/K 3. Consider the combustion of propane gas: C3H8 (g) + 5 O2 (g) → 3 CO2 (g ...
Thermodynamics
... Every thermodynamic system exists in a particular state. A thermodynamic cycle occurs when a system is taken through a series of different states, and finally returned to its initial state. In the process of going through this cycle, the system may perform work on its surroundings, thereby acting as ...
... Every thermodynamic system exists in a particular state. A thermodynamic cycle occurs when a system is taken through a series of different states, and finally returned to its initial state. In the process of going through this cycle, the system may perform work on its surroundings, thereby acting as ...