Repetition Summary of last lecture Energy Cell Respiration
... In a c ti v e tra n s c ri p ti o n fa c to r ...
... In a c ti v e tra n s c ri p ti o n fa c to r ...
Krebs cycle - biology.org.uk
... joins with oxaloacetate, a four-carbon compound, to form citrate, a six-carbon compound 2 Citrate is decarboxylated (one molecule of CO 2 removed) and dehydrogenated (two hydrogen atoms removed) to form a five-carbon compound; and the hydrogen atoms are accepted by an NAD molecule, which gets reduce ...
... joins with oxaloacetate, a four-carbon compound, to form citrate, a six-carbon compound 2 Citrate is decarboxylated (one molecule of CO 2 removed) and dehydrogenated (two hydrogen atoms removed) to form a five-carbon compound; and the hydrogen atoms are accepted by an NAD molecule, which gets reduce ...
presentation source
... Chemiosmosis in mitochondria • Energy from electrons is harnessed by – NADH dehydrogenase complex – cytochrome c reductase complex – cytochrome c oxidase complex • the complexes pump protons against their gradient from the matrix into the inner membrane space • a strong diffusion gradient is set up ...
... Chemiosmosis in mitochondria • Energy from electrons is harnessed by – NADH dehydrogenase complex – cytochrome c reductase complex – cytochrome c oxidase complex • the complexes pump protons against their gradient from the matrix into the inner membrane space • a strong diffusion gradient is set up ...
Pyruvate to Acetyl Coenzyme A (Acetyl CoA)
... o Protons (H+) flow through ATP Synthase from the intermembrane space into the matrix. The flow of electrons releases enough energy to attach phosphate groups to ADP to generate ATP. Net Gain: 32 ATP o 2 electrons and 2 protons (H+) attach to ½ O2 to form water. Poisons and Cellular Respiration ...
... o Protons (H+) flow through ATP Synthase from the intermembrane space into the matrix. The flow of electrons releases enough energy to attach phosphate groups to ADP to generate ATP. Net Gain: 32 ATP o 2 electrons and 2 protons (H+) attach to ½ O2 to form water. Poisons and Cellular Respiration ...
Cellular Respiration
... • Step-wise degradation harvests more energy • Electrons are first passed to NAD+, then through the electron transport chain, then to oxygen ...
... • Step-wise degradation harvests more energy • Electrons are first passed to NAD+, then through the electron transport chain, then to oxygen ...
Chapter 9: Cellular Respiration
... • Enzymes ____________________ for the electron transport chain are located on the inner mitochondrial membrane. Several complexes are called __________________. • Electrons from NADH and FADH2 travel down the electron transport chain, ___________________________________________________ ____________ ...
... • Enzymes ____________________ for the electron transport chain are located on the inner mitochondrial membrane. Several complexes are called __________________. • Electrons from NADH and FADH2 travel down the electron transport chain, ___________________________________________________ ____________ ...
The Citric Acid Cycle - Rubin Risto Gulaboski
... Review of Glycolysis • What’s the point of glycolysis? – Traps glucose within the cell – Produces energy in the form of NADH and ATP with minimal energy input – Produces products that are used in other metabolic pathways within the cell ...
... Review of Glycolysis • What’s the point of glycolysis? – Traps glucose within the cell – Produces energy in the form of NADH and ATP with minimal energy input – Produces products that are used in other metabolic pathways within the cell ...
Respiration
... FADH2 None of these makes more ATP; they all produce the same amount During beta oxidation * a H+ gradient is created photophosphorylation makes ATP fatty acids are fed into the Krebs cycle NAD+ carriers are regenerated ...
... FADH2 None of these makes more ATP; they all produce the same amount During beta oxidation * a H+ gradient is created photophosphorylation makes ATP fatty acids are fed into the Krebs cycle NAD+ carriers are regenerated ...
Coenzymes
... Vitamin K (phylloquinone) • Required for synthesis of blood coagulation proteins • A coenzyme for mammalian carboxylases that convert glutamate to g-carboxyglutamate residues • Calcium binds to the g-carboxyGlu residues of these coagulation proteins which adhere to platelet surfaces • Vitamin K ana ...
... Vitamin K (phylloquinone) • Required for synthesis of blood coagulation proteins • A coenzyme for mammalian carboxylases that convert glutamate to g-carboxyglutamate residues • Calcium binds to the g-carboxyGlu residues of these coagulation proteins which adhere to platelet surfaces • Vitamin K ana ...
Lecture 21
... Isozymes: Enzymes that catalyze the same reaction but are different in their kinetic behavior Tissue specific Glucokinase- Liver controls blood glucose levels. Hexokinase in muscle - allosteric inhibition by ATP Hexokinase in brain - NO allosteric inhibition by ATP ...
... Isozymes: Enzymes that catalyze the same reaction but are different in their kinetic behavior Tissue specific Glucokinase- Liver controls blood glucose levels. Hexokinase in muscle - allosteric inhibition by ATP Hexokinase in brain - NO allosteric inhibition by ATP ...
Krebs and ETC
... In step 1, acetyl-CoA combines with oxaloacetate to form citrate. NAD+ is reduced to NADH in steps 3, 4 and 8. FAD is reduced to FADH2 in step 6. ATP if formed in step 5 by substrate-level phosphorylation. The phosphate group from succinylCoA is transferred to GDP, forming GTP, which then forms ATP. ...
... In step 1, acetyl-CoA combines with oxaloacetate to form citrate. NAD+ is reduced to NADH in steps 3, 4 and 8. FAD is reduced to FADH2 in step 6. ATP if formed in step 5 by substrate-level phosphorylation. The phosphate group from succinylCoA is transferred to GDP, forming GTP, which then forms ATP. ...
Krebs cycle - Groby Bio Page
... 2 Idea that it is used to link reactions (1); idea that energy is released as a result of the activity of one enzyme and used by another enzyme (1). ...
... 2 Idea that it is used to link reactions (1); idea that energy is released as a result of the activity of one enzyme and used by another enzyme (1). ...
Summary
... acetyl-CoA(C2H3O-CoA) + 3 NAD+ + FAD + GDP + Pi + 2H2O CoA-SH + 2 CO2 + 3 NADH + 3 H+ + FADH2 + GTP Include pyruvate oxidation: pyruvic acid(C3H4O3) + 4 NAD+ + FAD + GDP + Pi + 2H2O 3 CO2 + 4 NADH + 4 H+ + FADH2 + GTP ...
... acetyl-CoA(C2H3O-CoA) + 3 NAD+ + FAD + GDP + Pi + 2H2O CoA-SH + 2 CO2 + 3 NADH + 3 H+ + FADH2 + GTP Include pyruvate oxidation: pyruvic acid(C3H4O3) + 4 NAD+ + FAD + GDP + Pi + 2H2O 3 CO2 + 4 NADH + 4 H+ + FADH2 + GTP ...
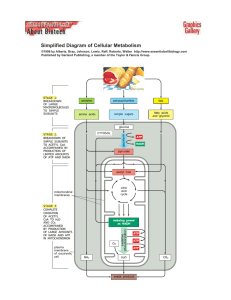
Simplified Diagram of Cellular Metabolism
... . http://www.essentialcellbiology.com Published by Garland Publishing, a member of the Taylor & Francis Group. ...
... . http://www.essentialcellbiology.com Published by Garland Publishing, a member of the Taylor & Francis Group. ...
1) Where does glycolysis occur in the cell
... c) the catabolism of citric acid to produce NADH, CO2, AND H+ d) the transfer of electrons form NADH to the electron transport chain e) the reduction of oxygen to form water. ...
... c) the catabolism of citric acid to produce NADH, CO2, AND H+ d) the transfer of electrons form NADH to the electron transport chain e) the reduction of oxygen to form water. ...
Lesson 4.2 Link Reaction and Krebs Cycle
... Krebs Cycle. Remember, respiration is all about releasing energy from your food. Oxidation releases energy. When a carbon compound is oxidised, coenzymes are reduced. The coenzymes involved are: NAD and FAD. Decarboxylation is the removal of CO2. Remember this: 665 and five 4’s. ...
... Krebs Cycle. Remember, respiration is all about releasing energy from your food. Oxidation releases energy. When a carbon compound is oxidised, coenzymes are reduced. The coenzymes involved are: NAD and FAD. Decarboxylation is the removal of CO2. Remember this: 665 and five 4’s. ...
Enzymes
... particular configuration of the active site into which the substrate molecules fit like a key, giving rise to the lock and key hypothesis. This hypothesis has been modified to the induced fit hypothesis, where it is thought that when a Substrate combines with an enzyme, it induces the enzyme structu ...
... particular configuration of the active site into which the substrate molecules fit like a key, giving rise to the lock and key hypothesis. This hypothesis has been modified to the induced fit hypothesis, where it is thought that when a Substrate combines with an enzyme, it induces the enzyme structu ...
Problem Set 1 - Berkeley MCB
... In humans, gluconeogenesis (A) can result in the conversion of protein into blood glucose. (B) helps to reduce blood glucose after a carbohydrate-rich meal. (C) is activated by the hormone insulin (D) is essential in the conversion of fatty acids to glucose. (E) requires the enzyme hexokinase. ...
... In humans, gluconeogenesis (A) can result in the conversion of protein into blood glucose. (B) helps to reduce blood glucose after a carbohydrate-rich meal. (C) is activated by the hormone insulin (D) is essential in the conversion of fatty acids to glucose. (E) requires the enzyme hexokinase. ...
Slide 1
... Metabolism is the sum total of all interactions between molecules within cell environments. The chemistry of life is organized into metabolic pathways. Metabolic pathways begin with a specific molecule, which is then altered in a series of defined steps to form a specific product. ...
... Metabolism is the sum total of all interactions between molecules within cell environments. The chemistry of life is organized into metabolic pathways. Metabolic pathways begin with a specific molecule, which is then altered in a series of defined steps to form a specific product. ...
Exam 3
... 15. What are the net end-products from glycolysis fed into the Krebs cycle and electron transport systems (ETS)? A. 2 NADH B. 2 Pyruvate C. 2ATP D. 2NADPH E. A & B. ...
... 15. What are the net end-products from glycolysis fed into the Krebs cycle and electron transport systems (ETS)? A. 2 NADH B. 2 Pyruvate C. 2ATP D. 2NADPH E. A & B. ...
Exam I Review - Iowa State University
... a. NAD+ is reduced to NADH during both glycolysis and the citric acid cycle. *b. NAD+ has more chemical potential energy than NADH. c. NAD+ can receive electrons for use in electron transport and oxidative phosphorylation. d. In the absence of NAD+, glycolysis cannot proceed. 162. The ATP made durin ...
... a. NAD+ is reduced to NADH during both glycolysis and the citric acid cycle. *b. NAD+ has more chemical potential energy than NADH. c. NAD+ can receive electrons for use in electron transport and oxidative phosphorylation. d. In the absence of NAD+, glycolysis cannot proceed. 162. The ATP made durin ...
Exam I Review - Iowa State University
... a. NAD+ is reduced to NADH during both glycolysis and the citric acid cycle. b. NAD+ has more chemical potential energy than NADH. c. NAD+ can receive electrons for use in electron transport and oxidative phosphorylation. d. In the absence of NAD+, glycolysis cannot proceed. 162. The ATP made during ...
... a. NAD+ is reduced to NADH during both glycolysis and the citric acid cycle. b. NAD+ has more chemical potential energy than NADH. c. NAD+ can receive electrons for use in electron transport and oxidative phosphorylation. d. In the absence of NAD+, glycolysis cannot proceed. 162. The ATP made during ...
Cellular Respiration: the details
... molecule broken down into 2 pyruvate molecules; in cytoplasm ...
... molecule broken down into 2 pyruvate molecules; in cytoplasm ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.