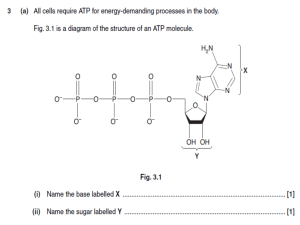
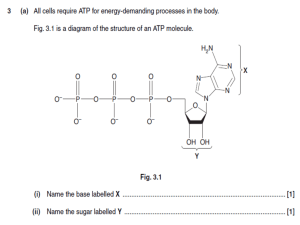
Chapter 19
... • a-Ketoglutarate dehydrogenase complex: inhibited by ATP, NADH, and succinyl CoA; activated by ADP and NAD+. ...
... • a-Ketoglutarate dehydrogenase complex: inhibited by ATP, NADH, and succinyl CoA; activated by ADP and NAD+. ...
lec33_F2015
... ii) Pyruvate can be converted to alanine in a one-step transaminase reaction. iii) Pyruvate can be used to make oxaloacetate, to replace the carbons that are removed from the citric acid cycle by anabolic processes (this reaction is the first step in gluconeogenesis). Cooperation between muscle and ...
... ii) Pyruvate can be converted to alanine in a one-step transaminase reaction. iii) Pyruvate can be used to make oxaloacetate, to replace the carbons that are removed from the citric acid cycle by anabolic processes (this reaction is the first step in gluconeogenesis). Cooperation between muscle and ...
Cellular Respiration
... Something is reduced if it gains an electron. Oxidation. Something is oxidized if it loses an electron. ...
... Something is reduced if it gains an electron. Oxidation. Something is oxidized if it loses an electron. ...
Lecture 7-enzymes 3
... Most enzymes are named for their substrates and for the type of reactions they catalyze, with the suffix “ase” added For example; ATPase is an enzyme that breaks down ATP, whereas ATP synthase is an enzyme that synthesizes ATP Some enzymes have common names that provide little information abou ...
... Most enzymes are named for their substrates and for the type of reactions they catalyze, with the suffix “ase” added For example; ATPase is an enzyme that breaks down ATP, whereas ATP synthase is an enzyme that synthesizes ATP Some enzymes have common names that provide little information abou ...
Cellular Respiration - Science with Ms. Wood!
... The summary equation of cellular respiration. The difference between fermentation and cellular respiration. The role of glycolysis in oxidizing glucose to two molecules of pyruvate The process that brings pyruvate from the cytosol into the mitochondria and introduces it into the citric acid cyc ...
... The summary equation of cellular respiration. The difference between fermentation and cellular respiration. The role of glycolysis in oxidizing glucose to two molecules of pyruvate The process that brings pyruvate from the cytosol into the mitochondria and introduces it into the citric acid cyc ...
Microbial Metabolism Notes
... (i) O2 is considered the final electron acceptor (c) redox energy is used to pump H+ into the cell (i) creates a higher concentration in ICF (d) H+ is moved out through ATPsynthase creating ATP as it moves out (e) each NADH has enough energy to produce 3 ATP and each FADH2 can produce 2 (i) 30 ATP f ...
... (i) O2 is considered the final electron acceptor (c) redox energy is used to pump H+ into the cell (i) creates a higher concentration in ICF (d) H+ is moved out through ATPsynthase creating ATP as it moves out (e) each NADH has enough energy to produce 3 ATP and each FADH2 can produce 2 (i) 30 ATP f ...
SI Session 10-13-14 The molecule that functions as the reducing
... Why does the oxidation of organic compounds by molecular oxygen to produce CO2 and water release free energy? A) The covalent bonds in organic molecules are higher energy bonds than those in water and carbon dioxide. B) Electrons are being moved from atoms that have a lower affinity for electrons (s ...
... Why does the oxidation of organic compounds by molecular oxygen to produce CO2 and water release free energy? A) The covalent bonds in organic molecules are higher energy bonds than those in water and carbon dioxide. B) Electrons are being moved from atoms that have a lower affinity for electrons (s ...
Aerobic respiration
... • Oxygen is the final electron acceptor! • Oxygen combines with 2H+ and two electrons to form H2O! • Oxygen keeps the electrons moving through the chain! Without oxygen the electron transport chain would stop! No ATP would be generated! ...
... • Oxygen is the final electron acceptor! • Oxygen combines with 2H+ and two electrons to form H2O! • Oxygen keeps the electrons moving through the chain! Without oxygen the electron transport chain would stop! No ATP would be generated! ...
Cell Metabolism Review
... NADH and FADH2 to the Electron Transport Chain (ETC) where additional molecules of ATP are produced from the oxidation steps of the ETC (~34 additional ATPs per original molecule of glucose). Plants and some bacteria can harvest light energy and convert it to a form that is usable by other living or ...
... NADH and FADH2 to the Electron Transport Chain (ETC) where additional molecules of ATP are produced from the oxidation steps of the ETC (~34 additional ATPs per original molecule of glucose). Plants and some bacteria can harvest light energy and convert it to a form that is usable by other living or ...
untitled file - Blue Earth Area Schools
... • NADH donates the high energy electron back to pyruvate to form either lactic acid or ethanol and CO2 • Then NAD+ is recycled and glycolysis can ...
... • NADH donates the high energy electron back to pyruvate to form either lactic acid or ethanol and CO2 • Then NAD+ is recycled and glycolysis can ...
ANSWERS TO REVIEW QUESTIONS – CHAPTER 03
... What is the basis of the specificity of enzyme action? Explain in terms of enzyme structure why boiling inactivates enzymes. (pp. 60–65) An enzyme is a catalyst produced by the protein production machinery of a cell. It acts by increasing the rate of particular chemical reactions. The basis for enzy ...
... What is the basis of the specificity of enzyme action? Explain in terms of enzyme structure why boiling inactivates enzymes. (pp. 60–65) An enzyme is a catalyst produced by the protein production machinery of a cell. It acts by increasing the rate of particular chemical reactions. The basis for enzy ...
Key Terms:
... H+ gradient drives ATP synthesis Glycolysis is universal, anaerobic and cytosolic 2 ATP in; 4 ATP out & 2 reduced coenzymes glucose (six carbons, C6) 2 moleucles of pyruvate (three carbons, C3) Citric Acid Cycle, in the mitochondria Pyruvate crosses into mitochondrial matrix and is converted to ac ...
... H+ gradient drives ATP synthesis Glycolysis is universal, anaerobic and cytosolic 2 ATP in; 4 ATP out & 2 reduced coenzymes glucose (six carbons, C6) 2 moleucles of pyruvate (three carbons, C3) Citric Acid Cycle, in the mitochondria Pyruvate crosses into mitochondrial matrix and is converted to ac ...
Part 2
... • First the carboxyl group is split off of the 2 pyruvates as carbon dioxide • Then remaining two-carbon acetyl fragment is oxidized and electrons transferred to NAD+ making NADH • Finally, the oxidized two-carbon acetyl group is attached to coenzyme A • Creates acetyl CoA ...
... • First the carboxyl group is split off of the 2 pyruvates as carbon dioxide • Then remaining two-carbon acetyl fragment is oxidized and electrons transferred to NAD+ making NADH • Finally, the oxidized two-carbon acetyl group is attached to coenzyme A • Creates acetyl CoA ...
1. Which of the following is not a feature of scientific hypotheses? A
... C) Metabolic pathways in eukaryotes occur in the cytoplasm. D) Metabolic pathways vary from organism to organism. E) Each metabolic pathway is regulated by specific enzymes. ...
... C) Metabolic pathways in eukaryotes occur in the cytoplasm. D) Metabolic pathways vary from organism to organism. E) Each metabolic pathway is regulated by specific enzymes. ...
1. Metabolic pathways 2. Basic enzyme kinetics 3. Metabolic
... » Electrons are transported from NADH & FADH through the electron transport chain to oxygen » Electron transport causes protons to be released into the intermembrane space » These electrons can be transported back into mitochondrial matrix by a proton conducting ATP-synthase » The detailed mechanist ...
... » Electrons are transported from NADH & FADH through the electron transport chain to oxygen » Electron transport causes protons to be released into the intermembrane space » These electrons can be transported back into mitochondrial matrix by a proton conducting ATP-synthase » The detailed mechanist ...
Cell Respiration
... to safely move Hydrogen’s electron and proton power (proton motive force) ultimately to ATP Synthase. • Ubiquinone, Cytochrome C, NADH reductase • Use the proton motive force to make even more ATP . Many shuttle stations due to the folding of the cristae • Will use oxygen to drive the movement of hy ...
... to safely move Hydrogen’s electron and proton power (proton motive force) ultimately to ATP Synthase. • Ubiquinone, Cytochrome C, NADH reductase • Use the proton motive force to make even more ATP . Many shuttle stations due to the folding of the cristae • Will use oxygen to drive the movement of hy ...
Problem Set 5 (Due February 25th) 1. Show how glucose can be
... apologize if this gave you a headache. e. Figure 5 has a lot of important information. i. What does this figure tell us about Glucose-1-phosphate and AMP – are they activators or inhibitors? They are activators of the enzyme. Does this make sense based on the role of this enzyme? Yes, a lot of AMP s ...
... apologize if this gave you a headache. e. Figure 5 has a lot of important information. i. What does this figure tell us about Glucose-1-phosphate and AMP – are they activators or inhibitors? They are activators of the enzyme. Does this make sense based on the role of this enzyme? Yes, a lot of AMP s ...
U4L21 fuel oxidation - The University of Sydney
... • FA is carried in blood on albumin – Several binding sites for FA ...
... • FA is carried in blood on albumin – Several binding sites for FA ...
Answer Key
... What is the final electron acceptor at the end of Electron Transport? oxygen What happens to the NADH’s produced during glycolysis and Krebs cycle? If oxygen is present, goes to ETC. No oxygen onto fermentation. What high energy electron carriers are used in respiration? NAD+ and FAD How are these d ...
... What is the final electron acceptor at the end of Electron Transport? oxygen What happens to the NADH’s produced during glycolysis and Krebs cycle? If oxygen is present, goes to ETC. No oxygen onto fermentation. What high energy electron carriers are used in respiration? NAD+ and FAD How are these d ...
Chapter 5: Self Test
... b. The cells will utilize oxygen more rapidly. c. The rate of the Krebs cycle reactions will increase. d. Electron transport will increase. e. The rate of fermentation will increase. 7. When oxygen is present, a. most cells utilize aerobic cellular respiration. b. most animal cells will carry on fer ...
... b. The cells will utilize oxygen more rapidly. c. The rate of the Krebs cycle reactions will increase. d. Electron transport will increase. e. The rate of fermentation will increase. 7. When oxygen is present, a. most cells utilize aerobic cellular respiration. b. most animal cells will carry on fer ...
Anaerobic Respiration
... • This is the hydrogen acceptor, not pyruvate. • Pyruvate is produced as a result of glycolysis but is decarboxylated to ethanal • Ethanal is reduced to ethanol. This occurs because of alcohol dehydrogenase enzymes. • To stop a build up of ethanol in human liver cells, alcohol dehydrogenase adds hyd ...
... • This is the hydrogen acceptor, not pyruvate. • Pyruvate is produced as a result of glycolysis but is decarboxylated to ethanal • Ethanal is reduced to ethanol. This occurs because of alcohol dehydrogenase enzymes. • To stop a build up of ethanol in human liver cells, alcohol dehydrogenase adds hyd ...
Aerobic Respiration
... • A complex organic molecule containing a nucleotide (adenine and ribose) with a B group vitamin (pantothenic acid) • Acts as a carrier of acetyl groups to the Kreb’s cycle ...
... • A complex organic molecule containing a nucleotide (adenine and ribose) with a B group vitamin (pantothenic acid) • Acts as a carrier of acetyl groups to the Kreb’s cycle ...
Anaerobic Respiration
... • This is the hydrogen acceptor, not pyruvate. • Pyruvate is produced as a result of glycolysis but is decarboxylated to ethanal • Ethanal is reduced to ethanol. This occurs because of alcohol dehydrogenase enzymes. • To stop a build up of ethanol in human liver cells, alcohol dehydrogenase adds hyd ...
... • This is the hydrogen acceptor, not pyruvate. • Pyruvate is produced as a result of glycolysis but is decarboxylated to ethanal • Ethanal is reduced to ethanol. This occurs because of alcohol dehydrogenase enzymes. • To stop a build up of ethanol in human liver cells, alcohol dehydrogenase adds hyd ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.