* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download SI Session 10-13-14 The molecule that functions as the reducing
Basal metabolic rate wikipedia , lookup
Metalloprotein wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Phosphorylation wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biochemistry wikipedia , lookup
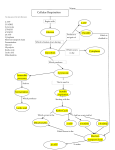
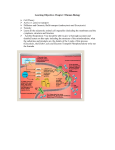
SI Session 10-13-14 The molecule that functions as the reducing agent (electron donor) in a redox or oxidationreduction reaction A) gains electrons and gains energy. B) loses electrons and loses energy. C) gains electrons and loses energy. D) loses electrons and gains energy. E) neither gains nor loses electrons, but gains or loses energy. Why does the oxidation of organic compounds by molecular oxygen to produce CO2 and water release free energy? A) The covalent bonds in organic molecules are higher energy bonds than those in water and carbon dioxide. B) Electrons are being moved from atoms that have a lower affinity for electrons (such as C) to atoms with a higher affinity for electrons (such as O). C) The oxidation of organic compounds can be used to make ATP. D) The electrons have a higher potential energy when associated with water and CO2 than they do in organic compounds. E) The covalent bond in O2 is unstable and easily broken by electrons from organic molecules. T/F NAD+ has more chemical energy than NADH. Where does glycolysis take place? Does it require oxygen? The ATP made during glycolysis is generated by A) substrate-level phosphorylation. B) electron transport. C) photophosphorylation. D) chemiosmosis. E) oxidation of NADH to NAD+. In order for NAD+ to remove electrons from glucose or other organic molecules, which of the following must be true? A) The organic molecule or glucose must be negatively charged in order to reduce the positively charged NAD+. B) Oxygen must be present to oxidize the NADH produced back to NAD+. C) The free energy liberated when electrons are removed from the organic molecules must be greater than the energy required to give the electrons to NAD+. D) A and B are both correct. E) A, B, and C are all correct. Tell me the end NET products of glycolysis. What holds the MOST energy? A molecule that is phosphorylated A) has an increased chemical reactivity; it is primed to do cellular work. B) has a decreased chemical reactivity; it is less likely to provide energy for cellular work. C) has been oxidized as a result of a redox reaction involving the gain of an inorganic phosphate. D) has been reduced as a result of a redox reaction involving the loss of an inorganic phosphate. E) has less energy than before its phosphorylation and therefore less energy for cellular work. Products for oxidation of one pyruvate? Where does acetyl CoA accumulate? Starting with one molecule of isocitrate and ending with fumarate, what is the maximum number of ATP molecules that could be made through substrate-level phosphorylation? A) 1 B) 2 C) 11 D) 12 E) 24 Carbon skeletons for amino acid biosynthesis are supplied by intermediates of the citric acid cycle. Which intermediate would supply the carbon skeleton for synthesis of a fivecarbon amino acid? Assuming you get 3 ATP for NADH and 2 ATP for FADH2 in oxidative phosphorylation, how many ATP molecules can be formed SOLELY from oxidative phosphorylation if you start with one molecule of citrate and end with oxaloacetate? Making the same assumption as above, how many ATP can you make through substratelevel phosphorylation AND oxidative phosphorylation if you start with 3 molecules of succinyl CoA and ended with oxaloacetate? Starting with citrate, how many of the following would be produced with three turns of the citric acid cycle? A) 1 ATP, 2 CO2, 3 NADH, and 1 FADH2 B) 2 ATP, 2 CO2, 1 NADH, and 3 FADH2 C) 3 ATP, 3 CO2, 3 NADH, and 3 FADH2 D) 3 ATP, 6 CO2, 9 NADH, and 3 FADH2 E) 38 ATP, 6 CO2, 3 NADH, and 12 FADH2 Where do the catabolic products of fatty acid breakdown enter into the citric acid cycle? A) pyruvate B) malate or fumarate C) acetyl CoA D) α-ketoglutarate E) succinyl CoA Where can you find the electron transport chain? Where do you find H+ ions as energy is released by the electron transport chain? Is this the direct energy source that drives ATP synthesis in oxidative phosphorylation?