TCA Cycle Handout 1
... enzymes essential for energy production through aerobic respiration, and, like glycolysis, arose early in evolution. This pathway is also an important source of biosynthetic building blocks used in gluconeogenesis, amino acid biosynthesis, and fatty acid biosynthesis. The Krebs cycle takes place in ...
... enzymes essential for energy production through aerobic respiration, and, like glycolysis, arose early in evolution. This pathway is also an important source of biosynthetic building blocks used in gluconeogenesis, amino acid biosynthesis, and fatty acid biosynthesis. The Krebs cycle takes place in ...
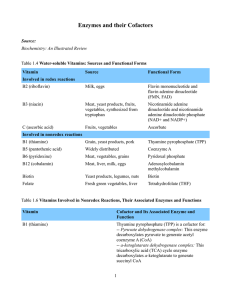
Enzymes and their Cofactors Source: Biochemistry: An Illustrated
... cofactors for: -- Methylmalonyl CoA mutase: This enzyme isomerizes methylmalonyl CoA into succinyl CoA in a reaction that is part of the pathway that degrades odd-numbered fatty acids -- Methionine synthase/homocysteine methyltransferase: This enzyme transfers a methyl group from 5-methyltetrahydrof ...
... cofactors for: -- Methylmalonyl CoA mutase: This enzyme isomerizes methylmalonyl CoA into succinyl CoA in a reaction that is part of the pathway that degrades odd-numbered fatty acids -- Methionine synthase/homocysteine methyltransferase: This enzyme transfers a methyl group from 5-methyltetrahydrof ...
AA lecture 2 urea cycle
... H20 + fumarate + aa + NAD+ aspartate + -keto acid + NADH + H+ then aspartate + NH4+ + HCO3- + 3 ATP urea + fumarate + 2 H20 + 2 ADP + AMP + 4 Pi + H+ Four high energy phosphate bond equivalents are used for these reactions (- 4 ~P). Two NADH are produced. ...
... H20 + fumarate + aa + NAD+ aspartate + -keto acid + NADH + H+ then aspartate + NH4+ + HCO3- + 3 ATP urea + fumarate + 2 H20 + 2 ADP + AMP + 4 Pi + H+ Four high energy phosphate bond equivalents are used for these reactions (- 4 ~P). Two NADH are produced. ...
The Krebs Cycle - County Central High School
... Why is this organelle so important for cellular respiration? It is important because it is where 3 of the stages occur and it is the power-house of the cell creating large quantities of ATP ...
... Why is this organelle so important for cellular respiration? It is important because it is where 3 of the stages occur and it is the power-house of the cell creating large quantities of ATP ...
TM corrigé (mars 2015) - Louis Morisod
... two phosphate groups. Otto Warburg first identified it in the 1930’s, and its functions were intensively investigated during the following years (1). It is a metabolite found in all li ...
... two phosphate groups. Otto Warburg first identified it in the 1930’s, and its functions were intensively investigated during the following years (1). It is a metabolite found in all li ...
CELLULAR RESPIRATION Aerobic Cellular Respiration
... process used by cells to release energy from organic molecules (food) such as glucose ~this energy is stored in the molecule ATP ~ ATP = adenosine triphosphate or A~P~P~P ~ the process is controlled by enzymes ...
... process used by cells to release energy from organic molecules (food) such as glucose ~this energy is stored in the molecule ATP ~ ATP = adenosine triphosphate or A~P~P~P ~ the process is controlled by enzymes ...
2.277 December 2005 Final Exam
... A) Aquaporins use the energy of ATP to transport 2 Na+ into a cell and 3 K+ out of a cell. B) The fluid mosaic model of a membrane assumes that lipids travel rapidly around the bilayer but proteins are fixed and unable to move. C) Glucose permease is a 12 α-helical protein that uses the energy of AT ...
... A) Aquaporins use the energy of ATP to transport 2 Na+ into a cell and 3 K+ out of a cell. B) The fluid mosaic model of a membrane assumes that lipids travel rapidly around the bilayer but proteins are fixed and unable to move. C) Glucose permease is a 12 α-helical protein that uses the energy of AT ...
Cell Respiration Student Notes
... • Enzymes – ________________ the rate of chemical reactions • Substrates – molecules ________________ with enzymes • Only one small part of an enzyme, called the _______________, reacts with the substrate(s). • Active site may undergo a slight change in ____________ in order to fit with the substra ...
... • Enzymes – ________________ the rate of chemical reactions • Substrates – molecules ________________ with enzymes • Only one small part of an enzyme, called the _______________, reacts with the substrate(s). • Active site may undergo a slight change in ____________ in order to fit with the substra ...
Respiration II
... In terms of redox chemistry where chemistry, where is the “action” (the big potential (the big potential energy drops) in glucose ...
... In terms of redox chemistry where chemistry, where is the “action” (the big potential (the big potential energy drops) in glucose ...
Comparing Fermentation with Anaerobic and
... All use glycolysis (net ATP 2) to oxidize glucose and harvest chemical energy of food In all three, NAD is the oxidizing agent that accepts electrons during glycolysis The processes have different final electron acceptors: an organic molecule (such as pyruvate or acetaldehyde) in fermentati ...
... All use glycolysis (net ATP 2) to oxidize glucose and harvest chemical energy of food In all three, NAD is the oxidizing agent that accepts electrons during glycolysis The processes have different final electron acceptors: an organic molecule (such as pyruvate or acetaldehyde) in fermentati ...
APBioReview
... Movement of electrons through the ETC is accompanied by a protein pumping mechanism that sets up an energy gradient consisting of hydrogen ions (protons) across the inner mitochondrial membrane. These are pumped from the inner mitochondrial matrix to the outer compartment. As they flow back through ...
... Movement of electrons through the ETC is accompanied by a protein pumping mechanism that sets up an energy gradient consisting of hydrogen ions (protons) across the inner mitochondrial membrane. These are pumped from the inner mitochondrial matrix to the outer compartment. As they flow back through ...
CELLULAR RESPIRATION
... 3) The same enzyme sometimes works for both the forward and reverse reactions, but not always 4) Each type of enzyme recognizes and binds to only certain substrates ...
... 3) The same enzyme sometimes works for both the forward and reverse reactions, but not always 4) Each type of enzyme recognizes and binds to only certain substrates ...
Photosynthesis: dark reactions
... • some 3PGA (phosphoglyceric acid -- product of first step in Calvin Cycle) is transported into the cytosol and used to make amino acids • G-3-P (glyceraldehyde 3-P) is used to make fructose with is in turn used to make other sugars and starch • some fructose is converted into glucose; molecular of ...
... • some 3PGA (phosphoglyceric acid -- product of first step in Calvin Cycle) is transported into the cytosol and used to make amino acids • G-3-P (glyceraldehyde 3-P) is used to make fructose with is in turn used to make other sugars and starch • some fructose is converted into glucose; molecular of ...
rll 24.5 The citric ocid cycle
... This is what happens in the citric acid cycle: 1. Acetyl CoA and oxaloacetatecombine to form citrate. 2. Citric acid eventually loses two carbon atoms as carbon dioxide. The carbons in the two molecules of carbon dioxide are not the same carbons that entered the citric acid cycle as acetyl groups of ...
... This is what happens in the citric acid cycle: 1. Acetyl CoA and oxaloacetatecombine to form citrate. 2. Citric acid eventually loses two carbon atoms as carbon dioxide. The carbons in the two molecules of carbon dioxide are not the same carbons that entered the citric acid cycle as acetyl groups of ...
Amino acid chains may form helices as parts of the corresponding
... How ATP makes reactions possible, in this case: ...
... How ATP makes reactions possible, in this case: ...
Overview of Metaboli.. - Frozen Crocus Productions
... production in mitochondria, produces NADH (electrons) for ETC in mitochondria, anaerobic production of ATP MK & CPK: anaerobic production of ATP TCA: accepts acetyl-CoA for citrate synthesis, production of NADH (electrons) & TCAintermediates can be used for synthesis of lipids, DNA, RNA, many amino ...
... production in mitochondria, produces NADH (electrons) for ETC in mitochondria, anaerobic production of ATP MK & CPK: anaerobic production of ATP TCA: accepts acetyl-CoA for citrate synthesis, production of NADH (electrons) & TCAintermediates can be used for synthesis of lipids, DNA, RNA, many amino ...
Part 2 - Saddleback College
... oxidative phosphorylation to yield more (Think: Disney dollars - can only get this energy converted to ATP at the ETC) ...
... oxidative phosphorylation to yield more (Think: Disney dollars - can only get this energy converted to ATP at the ETC) ...
Exam 1 2007 - chem.uwec.edu
... a and d 5. What two 3-carbon molecules are generated by the cleavage of fructose-1,6bisphosphate? A) glyceraldehyde-3-phosphate and 3-phosphoglycerate B) glyceraldehyde-3-phosphate and dihydroxyacetone phosphate C) pyruvate and phosphoenolpyruvate D) enolase and 2-phosphoglycerate E) glyceraldehyde- ...
... a and d 5. What two 3-carbon molecules are generated by the cleavage of fructose-1,6bisphosphate? A) glyceraldehyde-3-phosphate and 3-phosphoglycerate B) glyceraldehyde-3-phosphate and dihydroxyacetone phosphate C) pyruvate and phosphoenolpyruvate D) enolase and 2-phosphoglycerate E) glyceraldehyde- ...
3. Biotechnological Importance of MO - Copy
... sorbitol to sorbose, synthesis of steroid hormones and certain amino acids ...
... sorbitol to sorbose, synthesis of steroid hormones and certain amino acids ...
Respiration
... 2. Use the following terms correctly in a sentence: redox reactions, oxidation, reduction, reducing agent and oxidizing agent. ...
... 2. Use the following terms correctly in a sentence: redox reactions, oxidation, reduction, reducing agent and oxidizing agent. ...
PowerPoint 演示文稿
... • A variant of TCA for plants and bacteria Acetate-based growth - net synthesis of carbohydrates and other intermediates from acetate - is not possible with TCA Glyoxylate cycle offers a solution for plants and some bacteria and algae The CO2-evolving steps are bypassed and an extra acetate is ut ...
... • A variant of TCA for plants and bacteria Acetate-based growth - net synthesis of carbohydrates and other intermediates from acetate - is not possible with TCA Glyoxylate cycle offers a solution for plants and some bacteria and algae The CO2-evolving steps are bypassed and an extra acetate is ut ...
6.8-6.10 Citric acid cycle and Oxidative phosphorylation
... • Pyruvate does not enter the citric acid cycle, but undergoes some chemical grooming in which – a carboxyl group is removed and given off as CO2, – the two-carbon compound remaining is oxidized while a molecule of NAD+ is reduced to NADH, – coenzyme A joins with the two-carbon group to form acetyl ...
... • Pyruvate does not enter the citric acid cycle, but undergoes some chemical grooming in which – a carboxyl group is removed and given off as CO2, – the two-carbon compound remaining is oxidized while a molecule of NAD+ is reduced to NADH, – coenzyme A joins with the two-carbon group to form acetyl ...
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. The compound is a dinucleotide, because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide. Nicotinamide adenine dinucleotide exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively.In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications. Because of the importance of these functions, the enzymes involved in NAD metabolism are targets for drug discovery.In organisms, NAD can be synthesized from simple building-blocks (de novo) from the amino acids tryptophan or aspartic acid. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin called niacin. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate (NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.Although NAD+ is written with a superscript plus sign because of the formal charge on a particular nitrogen atom, at physiological pH for the most part it is actually a singly charged anion (charge of minus 1), while NADH is a doubly charged anion.