* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Energy Production
Nicotinamide adenine dinucleotide wikipedia , lookup
Metalloprotein wikipedia , lookup
Basal metabolic rate wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
Light-dependent reactions wikipedia , lookup
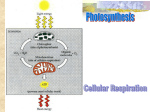
Microbial Metabolism Energy Production Energy production Nutrient molecules have energy associated with the electrons that form bonds between atoms Catabolic reactions oxidize nutrients by removing electrons and concentrate their energy into the bonds of ATP ATP has “high energy” or unstable bonds which allows the energy to be released quickly and easily. ATP ATP generation Cells use oxidation-reduction (redox) reactions in catabolism to extract energy from nutrient molecules This energy is trapped by the generation of ATP by phosphorylation of ADP Oxidation-reduction reactions Oxidation is the removal of electrons from a molecule Reduction is the gaining of electrons by a molecule Oxidation and reduction reactions are always coupled (redox reaction) Many catabolic oxidationreduction reactions are also dehydrogenation reactions The removal of electrons also means the removal of hydrogen atoms [i.e., not just an electron but also a proton (H+)] These are transferred to an “electron carrier” Electron carriers 2H+ + 2e- In catabolic reactions, energy is extracted from molecules in the form of electrons, which are transferred, along with H+ ions, to electron carriers like NAD+. NAD NADH+ + H+ Mechanisms of ATP generation Substrate-level phosphorylation Oxidative phosphorylation Photophosphorylation Substrate-level phosphoryation ATP is generated when a high-energy phosphate is transferred directly to ADP from a phosphorylated substrate Oxidative phosphorylation Electrons are transferred from organic compounds through a series of electron carriers to O2 or other oxidized inorganic or organic molecules The sequence of electron carriers is called the electron transport chain The transfer of electrons from one carrier to the next generates energy which is used to make ATP from ADP by chemiosmosis Photophosphorylation Only occurs in photosynthetic cells which contain light trapping pigment such as chlorophyll Light causes chlorophyll to give up electrons Energy released by the transfer of electrons from chlorophyll to carrier molecules is used to generate ATP How do chemoheterotrophs generate energy? Sources of energy: carbohydrate, fat, protein, minerals. Most microorganisms oxidize carbohydrates as the major source of cellular energy Energy can also be derived from the oxidation of fats, proteins, and minerals. Carbohydrate catabolism Microbes use two general processes to generate energy from glucose Aerobic respiration Fermentation Both start with glycolysis (= Emden Meyerhoff pathway) Aerobic Respiration Glycolysis (Embden-Meyerhof) TCA cycle (Kreb’s cycle) Glucose is oxidized to pyruvic acid Pyruvic acid is oxidized to acetyl CoA Acetyl CoA is oxidized to CO2 Electron transport chain Reduced NADH and FADH2 from the above are oxidized through a series of redox reactions through an electron transport chain. Glycolysis Starting point for cellular respiration and fermentation. 10 step catabolic pathway Two stages Preparatory stage Energy conserving stage Glycolysis: preparatory stage Glucose ATP Hexokinase 2 ATPs are used Glucose is split to form 2 molecules of Glyceraldehyde3-phosphate ADP P Glucose 6-phosphate Phosphoglucoisomerase P Fructose 6-phosphate ATP Phosphofructokinase ADP P Dihydroxyacetone P phosphate P Fructose 1,6-diphosphate aldolase Triose phosphate isomerase P Glyceraldehyde 3-phosphate Glycolysis: energy conserving stage P NAD+ For each initial glucose molecule; 2 Glyceraldehyde3-phosphate oxidized to 2 Pyruvic acid 4 ATP produced 2 NADH produced NAD+ NADH Diphosphoglyceric acid P P P ADP P ADP Phosphoglycerokinase ATP ATP 3-phosphoglyceric acid P Phosphoglyceromutase P H2O 2-phosphoglyceric acid P P H2O Enolase P ATP P P Triose phosphate P dehydrogenase NADH ADP Glyceraldehyde 3-phosphate Phosphoenolpyruvic acid Pyruvate kinase Pyruvic acid P ADP ATP Summary of glycolysis Glucose (C6H12O6) is split and oxidized through a ten step pathway to two molecules of pyruvic acid (C3H4O3) Net gain of 2 ATP molecules, 4 from energy conserving phase (by substrate level phosphorylation) minus 2 from preparatory phase 2 NADH molecules produced Pyruvic acid can now undergo either fermentation or respiration Alternatives to glycolysis Many bacteria have an alternative pathway to glycolysis for the oxidation of glucose Entner-doudoroff reaction Phosphogluconate pathway Some bacteria oxidize inorganic compounds instead of glucose to get energy. (the Lithotrophs) Use of Inorganic ions as electron “SOURCES” (Lithotrophs) Bacteria, by Energy sources Phototrophs Chemotrophs Oxidize organic compounds for Energy: Chemoorganotrophs Oxidize inorganic compounds for Energy: Chemolithotrophs