* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download second exam2
Basal metabolic rate wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Signal transduction wikipedia , lookup
Mitochondrion wikipedia , lookup
Biosynthesis wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
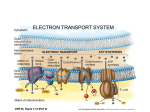
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Western blot wikipedia , lookup
Photosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biochemistry wikipedia , lookup
Biochem 462 Second Exam 3/14/2003 Note: the last several pages of the exam contain very useful information!! Multiple choice (45%) 1) There are three main points of regulation in glycolysis. They all have what in common? a) They all involve reactions before the 6-carbon backbone is split into two 3-carbon compounds. b) They all involve reactions where chemical oxidation of the sugar backbone takes place. c) They all involve reactions where there is a large negative free energy change. d) They all involve reactions that are coupled to the synthesis of ATP from ADP. 2) In the absence of oxygen, how is the NADH formed by the glyceraldehyde-3phosphate dehydrogenase reaction recycled to form NAD+? a) The NADH is oxidized to NAD+ during oxidative phophorylation. b) Pyruvate is reduced to lactate or ethanol and this is coupled to oxidation of NADH. c) The NADH is re-oxidized during the pyruvate dehydrogenase reaction. d) The NADH is all oxidized by anabolic reactions in the cell. 3) During the oxidation of glucose (glycolysis plus the TCA cycle), how many molecules of ATP and GTP combined are produced by substrate level phosphylation? a) 4 b) 5 c) 34 d) 38 4) Which of the following cofactors are important in the pyruvate dehydrogenase reaction. Circle all that are correct. You either get this all right or it is wrong. There is no partial credit. a) Coenzyme A b) NAD+ c) Lipoic Acid d) Thiamine pyrophosphate 5) What is the fate of the middle carbon (the keto carbon) of pyruvate when it enters the TCA cycle? a) It is lost as CO2 during the first round after entering the TCA cycle. b) It is lost as CO2 during the second round after entering the TCA cycle. c) It is lost as CO2 over a number of rounds after entering the TCA cycle, some fraction each round. d) It becomes permanently incorporated into one of the sugars involved in the TCA cycle. 6) What description best fits the term “saturated fat”? a) A triglyceride molecule in which the carbon-carbon bonds in the fatty acid chains are all single bonds. b) A triglyceride molecule in which the carbon-carbon bonds in the fatty acid chains are all double bonds. c) A tryglyceride molecule that contains the greatest possible number of fatty acid chains. d) A triglyceride molecule in which all of the double bonds have been reacted with water forming alcohols. 7) Phospholipids commonly serve which of the following functions (pick the best one)? a) They serve as cofactors for transfer of phosphate to ADP to form ATP. b) They are a component of the bilayers that make up biological membranes. c) They transport ions and other molecules in and out of cells. d) They are used to store energy as fat. 8) Which of the phrases below best describes the types of structural characteristics that have been described to date for integral membrane proteins? You must circle all the correct answers and no others in order to receive any credit. a) Globular proteins composed primarily of random coil structure with hydrophobic residues on the outside interacting with the lipid. b) Proteins made of alpha helical segments crossing the membrane connected by loops exposed to the solvent. c) Fibrous proteins with triple helical segments that cross the membrane connected by loops exposed to the solvent. d) Beta barrel proteins with the external amino acids being mostly hydrophobic and the internal amino acids being more hydrophilic. 9) Which of the following processes would be called facilitated diffusion: a) The nonspecific transport of a molecule across a membrane from high to low concentration. b) The transport of a molecule from low to high concentration across a membrane using a molecule-specific protein pump powered by ATP. c) The transport of a molecule from low to high concentrations across a membrane using a molecule-specific antiport or symport mechanism. d) The transport of a molecule from high to low concentrations across a membrane through molecule-specific protein channels. 10) The matrix of the mitochondrion refers to? a) The outer membrane. b) The space between the outer and inner membrane. c) The highly invaginated inner membrane. d) The space inside the inner membrane. 11) The redox reaction Malate + NAD+ oxaloacetate + NADH + H+ has a standard reduction potential of –0.154 V. Which of the following statements about this reaction is true? More than one may be true and you must check all of the right ones and no others in order to receive any credit. a) The reaction in the direction written is spontaneous when performed at standard state. b) The reaction in the direction written is not spontaneous when performed at standard state. c) The reaction in the direction written involves the oxidation of malate and the reduction of NAD+. d) The reaction in the direction written involves the reduction of malate and the oxidation of NAD+. 12) When your stomach is empty, what hormone is produced that acts as a short-term appetite stimulant? a) Leptin. b) Insulin. c) Ghrelin. d) PYY. Short Answer (40%) 13) What is the structure of the intermediate between Phosphoenolpyruvate and pyruvate? 14) Copy the structure of isocitrate from the TCA cycle in the back of the exam and circle the carbon that is being oxidized in the Isocitrate dehydrogenase reaction. 15) What is the structure of the intermediate in the Aconitase reaction? 16) Draw a biological membrane including the lipids, an integral transmembrane protein and a peripheral membrane protein. Label each of these components. This is just a cartoon like the pictures in the book. You do not need to draw the accurate chemical structures of any of the molecules. However, you do need to depict the right general locations and properties of these molecules. For example, your cartoon should show the positions of the head groups and tails of the lipids and how these lipids form a membrane. It should also show how the proteins are positioned relative to the membrane. 17) Lard (animal fat) is generally a solid at room temperature while plant oils are usually liquid at room temperature. What specific aspect of the chemical structure of the fatty acids involved contributes to this difference? The same chemical features present in lipids are also important in determining membrane fluidity. 18) Identify (by naming the enzyme involved) three of the major regulation points during the oxidation of pyruvate (this includes the reactions between pyruvate and the TCA cycle and the TCA cycle itself). Also name two of the major inhibitors that act at one or more of these sites. 19) We typically assign standard reduction potentials (often called midpoint potentials) to half reactions associated with oxidation and reduction of specific compounds (such as NAD+). For the reaction Malate + NAD+ oxaloacetate + NADH + H+ write down the two half reactions that make up the overall oxidation/reduction process. Please write them in the same direction as they would be written in a standard table (oxidized species on the left and reduced species on the right). Make sure the reactions are balanced. Hint: half of the answer to this question is contained in problem 20. Quantitative Understanding (15%) 20) In this problem, you will use what you know about oxidation/reduction reactions and about the coupling of chemical reactions to membrane potentials in order to calculate the maximum possible membrane potential that could be generated by NADH oxidation by oxygen and the maximum amount of ATP that could be generated from this process. ALL WORK MUST BE SHOWN FOR ANY CREDIT. a) 5 points. Consider the oxidation of NADH by oxygen (this is the reaction run in your body to power the oxidative phosphorylation of ADP to ATP). The reaction is: NADH + H+ + ½ O2 NAD+ + H2O The two half reactions you will need from the table in the book are: NAD+ + 2H+ + 2e- NADH + H+ E10 = -0.320 V + ½ O2 + 2H + 2e H2O E20 = +0.816 V What is the standard free energy for oxidation of a mole of NADH by oxygen? b) 5 points. If this was entirely converted into a membrane potential (), how large a potential would this free energy change theoretically be able to generate by serving as an energy source for pumping protons across the membrane? Assume that pH=7.0 on both sides of the membrane. I do not care about the sign of just the magnitude. (Note, this is just a theoretical thermodynamic calculation and will not result in the true membrane potential found in the cell.) c) 5 points. In your book it states the 3 ATP molecules can be made from the oxidation of one NADH molecule. What is the free energy associated with the overall process of coupling NADH oxidation to the production of 3 ATP molecules? Will this process be spontaneous? For the NADH oxidation reaction, just use the standard free energy change you calculated above without correction for actual concentrations. For the ATP hydrolysis, I want you to correct the standard free energy for the actual concentrations in the cell. Please use the following concentrations of ATP, ADP and Pi: 5 mM, 10 mM and 1 mM, respectively. The standard free energy change for the reaction, ATP + H2O ADP + Pi is –30.5 kJ/mole. Some Potentially Useful Equations and Constants: G = G0 + RTln(Q) G = zF + RTln([In]/[Out]) = 0 – (RT/nF)ln([Red]/[Ox]) RT at 295K = 2.45 kJ/mole F = 96.5 kJ/(mole volt)