* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 21 Biosynthetic Pathways
Nicotinamide adenine dinucleotide wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Proteolysis wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Genetic code wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
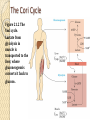
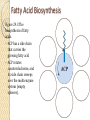
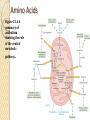
Chapter 21 Biosynthetic Pathways Introduction In most living organisms, the pathways by which a compound is synthesized are usually different from the pathways by which it is degraded; two reasons are 1. Flexibility: If a normal biosynthetic pathway is blocked, the organism can often use the reverse of the degradation pathway for synthesis. 2. Overcoming Le Châtelier’s principle: ◦ We can illustrate by this reaction: Introduction • Phosphorylase catalyzes both the forward and reverse reactions. • A large excess of phosphate would drive the reaction to the right; that is, drive the hydrolysis of glycogen. • To provide an alternative pathway for the synthesis of glycogen, even in the presence of excess phosphate: Most synthetic pathways are different from the degradation pathways. Most also differ in location and in energy requirements. Carbohydrate Biosynthesis We discuss the biosynthesis of carbohydrates under three headings: • Conversion of CO2 to glucose in plants. • Synthesis of glucose in animals and humans. • Conversion of glucose to other carbohydrates. Conversion of CO2 to carbohydrates in plants • Photosynthesis takes place in plants, green algae, and cyanobacteria. Synthesis of Glucose Gluconeogenesis: The synthesis of glucose from noncarbohydrate sources. • These sources are most commonly pyruvate, citric acid cycle intermediates, and glucogenic amino acids. • Gluconeogenesis is not the exact reversal of glycolysis; that is, pyruvate to glucose does not occur by reversing the steps of glucose to pyruvate. • There are three irreversible steps in glycolysis: ---Phosphoenolpyruvate to pyruvate + ATP. ---Fructose 6-phosphate to fructose 1,6-bisphosphate. ---Glucose to glucose 6-phosphate. • These three steps are reversed in gluconeogenesis, but by different reactions and using different enzymes. Other Carbohydrates • glycogenesis: The synthesis of glycogen from glucose. • The biosynthesis of other di-, oligo-, and polysaccharides also uses this common activation step to form an appropriate UDP derivative. The Cori Cycle Figure 21.2 The Cori cycle. Lactate from glycolysis in muscle is transported to the liver, where gluconeogensis converts it back to glucose. Fatty Acid Biosynthesis While degradation of fatty acids takes place in mitochondria, the majority of fatty acid synthesis takes place in the cytosol. These two pathways have in common that they both involve acetyl CoA. • Acetyl CoA is the end product of each spiral of b-oxidation. • Fatty acids are synthesized two carbon atoms at a time • The source of these two carbons is the acetyl group of acetyl CoA. The key to fatty acid synthesis is a multienzyme complex called acyl carrier protein, ACP-SH. Fatty Acid Biosynthesis Figure 29.3 The biosynthesis of fatty acids. • ACP has a side chain that carries the growing fatty acid • ACP rotates counterclockwise, and its side chain sweeps over the multienzyme system (empty spheres). Fatty Acid Biosynthesis ◦ Higher fatty acids, for example C18 (stearic acid), are obtained by addition of one or more additional C2 fragments by a different enzyme system. ◦ Unsaturated fatty acids are synthesized from saturated fatty acids by enzyme-catalyzed oxidation at the appropriate point on the hydrocarbon chain. ◦ The structure of NADP+ is the same as NAD+ except that there is an additional phosphate group on carbon 3’ of one of the ribose units. Membrane Lipids The two building blocks for the synthesis of membrane lipids are: • Activated fatty acids in the form of their acyl CoA derivatives. • Glycerol 1-phosphate, which is obtained by reduction of dihydroxyacetone phosphate (from glycolysis): CH2 -OH C=O + NADH + H+ 2CH2 -OPO3 Dihydroxyacetone phosphate CH2 -OH HO CH + NAD+ 2CH2 -OPO3 Glycerol 1-phosphate Membrane Lipids • Glycerol 1-phosphate combines with two acyl CoA molecules, which may be the same or different: CH2 -OH O + 2RC-S-CoA HO CH CH2 -OPO3 2Glycerol Acyl CoA 1-phosphate O CH2 -OCR O + 2CoA-SH RCO CH CH2 -OPO3 2A phosphatidate • To complete the synthesis of a phospholipid, an activated serine, choline, or ethanolamine is added to the phosphatidate by formation of a phosphoric ester. • Sphingolipids and glycolipids are assembled in similar fashion from the appropriate building blocks. Cholesterol All carbon atoms of cholesterol and of all steroids synthesized from it are derived from the two-carbon acetyl group of acetyl CoA. • Synthesis starts with reaction of three molecules of acetyl CoA to form the six-carbon compound 3-hydroxy-3-methylglutaryl CoA (HMG-CoA). • The enzyme HMG-CoA reductase then catalyzes the reduction of the thioester group to a primary alcohol. Cholesterol • In a series of steps requiring ATP, mevalonate undergoes phosporylation and decarboxylation to give the C5 compound, isopentenyl pyrophosphate. • This compound is a key building block for all steroids and bile acids. Cholesterol • Isopentenyl pyrophosphate (C5) is the building block for the synthesis of geranyl pyrophosphate (C10) and farnesyl pyrophosphate (C15). Amino Acids Most nonessential amino acids are synthesized from intermediates of either glycolysis or the citric acid cycle. • Glutamate, for example, is synthesized by amination and reduction of a-ketoglutarate, a citric acid cycle intermediate: Amino Acids • Glutamate in turn serves as an intermediate in the synthesis of several amino acids by the transfer of its amino group by transamination. Amino Acids • Figure 21.6 A summary of anabolism showing the role of the central metabolic pathway.