* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Doctoral thesis from the Department of Immunology, the Wenner-Gren Institute,... University, Stockholm, Sweden
Lymphopoiesis wikipedia , lookup
Infection control wikipedia , lookup
Herd immunity wikipedia , lookup
Neonatal infection wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Hepatitis B wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Adaptive immune system wikipedia , lookup
Immunocontraception wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Vaccination wikipedia , lookup
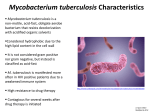
Tuberculosis wikipedia , lookup
DNA vaccination wikipedia , lookup
Innate immune system wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Doctoral thesis from the Department of Immunology, the Wenner-Gren Institute, Stockholm University, Stockholm, Sweden Mucosal immunity against mycobacterial infection Muhammad Jubayer Rahman Stockholm 2010 All previously published papers were reproduced with permission from the publishers Printed in Sweden by Universitetsservice AB, Stockholm 2010 Distributor: Stockholm University Library © Muhammad Jubayer Rahman, Stockholm 2010 ISBN 978-91-7447-081-9 2 "It is a good morning exercise for a research scientist to discard a pet hypothesis every day before breakfast - it keeps him/her young." Konrad Lorenz (1903 - 1989) 1973 Nobel Laureate in Medicine TO MY FAMILY 3 Summary This thesis aimed to the identification of immune biomarkers of mycobacterial infection for better diagnosis of tuberculosis (TB) and also focused on new vaccination strategies with a particular emphasis on the immune responses in the respiratory tract using murine models. Since the lung is the natural habitat for the M. tuberculosis, we reasoned that immune responses detected locally in the lungs would be good correlates of infection (Paper I). Likewise, immune responses induced in the respiratory tract following immunization would be more effective against mycobacterial infection. We showed that cytokines (IL-12, TNF, and IFN-γ) and cytokine receptors (sTNFR1 and sTNFR2) together with specific antibodies in the respiratory tract correlated better with the bacterial burden in the organs. In Paper II, we investigated the role of the BCG vaccination as a priming vaccine in a heterologous primeboost immunization protocol. The results showed that the neonatal BCG vaccination primed the immune system for a relevant antigen and showed a generalized adjuvant effect. Using this immunization protocol, protective immune responses in the lungs were generated independently of the route used for the booster immunization. In Paper III, We showed that exposure to mycobacterial antigens during the gestational period led to antigen transportation from the mother to the fetus and this resulted in an early priming of the fetal immune system. Immunization with the same antigen during the postnatal life increased antigen-specific recall IFN-γ responses and protection against infection. We examined the role of innate immunity for the induction of acquired immune responses upon immunization with mycobacterial antigens using TLR2 deficient mice (Paper IV). Our data indicated that suboptimal innate immune responses in the TLR2-/- mice might compromise the induction of acquired immune responses. Overall, the current findings suggested that a better understanding of the mucosal immunity would be useful for the improvement of diagnostic procedures and the development of efficient vaccines against TB. 4 LIST OF ARTICLES This thesis is based on the following original articles (manuscripts), which will be referred to by their Roman numerals: I. Arko-Mensah J*, Rahman MJ*, Julián E, Horner G, Singh M, Fernández C. Increased levels of immunological markers in the respiratory tract but not in serum correlate with active pulmonary mycobacterial infection in mice. Clin Microbiol Infect. 2009;15(8):777-86. * Authors contributed equally to this work II. Rahman MJ and Fernández C. Neonatal vaccination with Mycobacterium bovis BCG: potential effects as a priming agent shown in a heterologous prime-boost immunization protocol. Vaccine. 2009;27(30):4038-46. III. Rahman MJ, Dégano IR, Singh M and Fernández C. Influence of maternal gestational treatment with mycobacterial antigens on postnatal immunity in an experimental murine model. PLoS One. 2010;5(3):e9699. IV. Rahman MJ, Chuquimia OD, Singh M and Fernández C. Immunization with mycobacterial antigens: a role for innate immunity in antigen presentation. (Preliminary manuscript) 5 LIST OF ARTICLES (not included in this thesis) The following original articles are relevant but not included in this thesis. The articles will be cited by their Roman numerals: V. Arko-Mensah J*, Rahman MJ*, Dégano IR, Chuquimia OD, Fotio AL, Garcia I, Fernández C. Resistance to mycobacterial infection: a pattern of early immune responses leads to a better control of pulmonary infection in C57BL/6 compared with BALB/c mice. Vaccine. 2009;27(52):7418-27. * Authors contributed equally to this work VI. Olleros ML, Vesin D, Martinez-Soria E, Allenbach C, Tacchini-Cottier F, Pache JC, Marchal G, Rahman J, Fernández C, Izui S, Garcia I. Interleukin-12p40 overexpression promotes interleukin-12p70 and interleukin-23 formation but does not affect bacille Calmette-Guérin and Mycobacterium tuberculosis clearance. Immunology. 2007;122(3):350-61. 6 CONTENTS ................................................................................ Page Introduction------------------------------------------------------------------------- 10 Tuberculosis (TB)- a global health problem………………………………………10 Tuberculosis- clinical forms……………………………………………………… 10 Mycobacterium tuberculosis - the etiologic agent of TB………………………… 11 Host-pathogen interactions ------------------------------------------------------------------ 12 Mycobacterial survival strategies………………………………………………… 12 Adaptation of macrophages to infection ………………………………………….13 Susceptibility to infection………………………………………………………… 14 Immunology of TB------------------------------------------------------------------------------- 15 Innate immunity --------------------------------------------------------------------------- 16 Mycobacterial interactions with innate receptors………………………. 16 Macrophages……………………………………………………………. 19 Dendritic cells…………………………………………………………... 19 Epithelial cells…………………………………………………………...20 Natural killer (NK) cells ……………………………………………….. 21 Adaptive Immunity------------------------------------------------------------------------22 Cell-mediated immunity to mycobacteria………………………………. 22 CD4 T cells………………………………………………………………22 CD8 T cells………………………………………………………………23 Th17 cells……………………………………………………………….. 24 Gamma delta (γδ) T cells……………………………………………….. 25 Regulatory T cells (Treg)………………………………………………… 25 Humoral immunity……………………………………………………… 26 Cytokines and Chemokines…………………………………………………….. 28 Mucosal immunity in the respiratory tract……………………………………. 31 Vaccination against TB------------------------------------------------------------33 The BCG vaccine-------------------------------------------------------------------------- 33 Neonatal immunity and maternal-fetal interactions--------------------------------35 Failure of BCG and progress in new TB vaccine development-------------------37 Subunit vaccines…………………………………………………………38 DNA vaccines……………………………………………………………39 Auxotrophic vaccines…………………………………………………… 40 Recombinant (r) BCG……………………………………………………40 Combination vaccines (prime-boost)…………………………………… 41 Immune correlates of protection (multifunctional T cells)------------------------ 42 Route of vaccination (mucosal versus systemic)------------------------------------- 43 Diagnosis of TB--------------------------------------------------------------------- 46 Classical methods-------------------------------------------------------------------------- 46 Microscopy and culture…………………………………………………. 46 Tuberculin skin test…………………………………………………….. 47 Radiology or chest X-ray……………………………………………….. 47 New methods--------------------------------------------------------------------------------47 BACTEC radiometric system……………………………………………47 Microscopic-observation drug-susceptibility (MODS) assay………….. 48 Nucleic acid amplification assay……………………………………….. 48 Immune-based tests…………………………………………………….. 48 Biomarker (s) of infection………………………………………………. 51 The present study-------------------------------------------------------------------52 Aims------------------------------------------------------------------------------------------ 52 Methodology-------------------------------------------------------------------------------- 53 Results and discussion-------------------------------------------------------------------- 54 Paper I....................................................................................................... 54 Paper II…………………………………………………………………. 58 Paper III………………………………………………………………… 62 Paper IV………………………………………………………………… 65 Concluding remarks and future perspectives-------------------------------- 68 7 Acknowledgements----------------------------------------------------------------- 70 References----------------------------------------------------------------------------72 8 List of abbreviations AFB Ag APC BAL BALT BCG BCGBMM BCGAg CFP-10 CR CT CTL DC DC-SIGN ELISA ESAT-6 HIV i.n. IP-10 i.v. IFN-γ IL iNOS kDa LALT LAM MALT MDR MHC NALT nHBHA NO NOD PCR PI3P PknG PPD PRR rBCG RD rHBHA sTNFR TB TCR TLRs TNF WT XDR Acid fast bacilli Antigen Antigen presenting cell Broncho-alveolar lavage Bronchus-associated lymphoid tissue Bacillus Calmette-Guérin BCG infected bone-marrow derived macrophages antigen pulsed bone-marrow derived macrophages 10-kDa culture filtrate protein Complement receptor Cholera toxin Cytotoxic T lymphocyte Dendritic cell Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin Enzyme-linked immunosorbent assay 6-kDa early secretory antigenic target Human immunodeficiency virus Intranasal Interferon gamma-induced protein Intravenous Interferon gamma Interleukin Inducible nitric oxide synthase kiloDalton Larynx-associated lymphoid tissue Lipoarabinomannan Mucosa-associated lymphoid tissue Multidrug-resistant Major histocompatibility complex Nose-associated lymphoid tissue Heparin-binding hemagglutinin native Nitric oxide Nucleotide oligomerization domain Polymerase chain reaction phosphatidylinositol 3-phosphate protein kinase G Purified protein derivative Pattern recognition receptor Recombinant Bacillus Calmette-Guérin Region of deletion Heparin-binding hemagglutinin recombinant Soluble tumor necrosis factor receptor Tuberculosis T cell receptor Toll-like receptors Tumor necrosis factor Wild-type Extensively drug resistant 9 Introduction Tuberculosis- a global health problem: Tuberculosis (TB) is one of the leading infectious diseases in humans, caused by one of the most devious pathogens, Mycobacterium tuberculosis (M. tuberculosis). It is estimated that one third of the world’s population is infected with M. tuberculosis. Estimates from the World Health Organization indicate that 8-9 million new cases are reported every year (1). Even only 5-10% of the infected individuals progress to develop active TB, an annual instance of death due to TB is two million (2). Despite the fact that TB itself has been a threat for the world population, co-infection with human immunodeficiency virus (HIV) has further increased the risk of TB disease progression and deaths. Multidrug-resistant (MDR) TB has already been a problem in TBhistory and recently, extensively drug-resistant (XDR) TB has added more risk in the control of TB. At least 45 countries around the world have been identified with positive cases of XDR-TB (3). On the other side of the picture, several effective regimes have been developed over the past century to combat TB-pathogens including the TB-drugs and Mycobacterium bovis Calmitte Guerin (BCG) vaccine, which are likely the key components. Unfortunately, the control of TB is still a challenge, which simply translates into the urgent need of effective regimes available. Generally it is considered that vaccines are the most cost-efficient measures for the control of diseases if they work successfully. The BCG vaccine is the only recommended vaccine for humans against TB, developed in 1921, and found to be successful against only miliary TB in neonates and toddlers at a very low cost (4). Unfortunately, the BCG vaccine has been proven to be very inconsistent in protection against the most common form of adult pulmonary TB (5, 6). Tuberculosis- clinical forms: Lungs are the first habitat for the M. tuberculosis after being inhaled by a person. M. tuberculosis has a particular tropism for the lungs. Several possible outcomes can be seen when a person first encounters M. tuberculosis. First, the organisms can be immediately destroyed by the host innate immune barriers and the host remains uninfected. Second, people may contain TB germs in their body for their lifetime without showing clinical symptoms, which is called latent infection. Finally, only approximately 10% of the latently infected individuals may develop active TB with common clinical symptoms, i.e. chronic cough, fever, night sweats and weight loss. 10 There are two different types of TB in humans in general: pulmonary and extrapulmonary. Pulmonary TB is the most common form of TB that develops in the lungs. Manifestations of disease varied significantly from young children to adolescence to adults. Apparently, young children do not show classical symptoms of TB and that lead to difficulties in diagnosis and treatment. On the other hand, adult individuals show more clear symptoms in advanced pulmonary TB, which can primarily be diagnosed by X-ray radiography. Enlargement of mediastinal lymph nodes, bronchial obstruction may cause air trapping, hyperinflation, and even emphysema. A complete bronchial obstruction can be visualized by the typical radiographic shadows. Extrapulmonary TB develops in organs other than the lungs. Disseminated forms of TB, a type of extrapulmonary TB, are more common in young children than adults. Mycobacteria can be released into the blood stream or lymphatics from the primary infected site (lungs) and may cause disseminated TB in any parts of the body. Children, elderly people and patients with HIV infection are at greater risk of progressing disease more promptly due to their suboptimal immune function. The most common forms of disseminated TB are observed in kidney, bones, cervical lymph nodes, skin, stomach and meninges. Children at 26 months of age contract meningeal TB, which affect brain and central nervous system and can be fatal if leave untreated. M. tuberculosis - the etiologic agent of human TB: M. tuberculosis is a bacterial species, which belongs to the genus of Mycobacterium, family of Mycobacteriaceae and order of Actinomycetaceae. Robert Koch in 1882 first discovered M. tuberculosis and with further characterisation of this organism led to the understanding of causation of TB, which was later named Koch’s postulates in 1890. Mycobacteria are slow-growing, divide every 15-20 hours, aerobic, non-motile, nonsporulated rods. Most of the mycobacteria live and propagate in natural habitats such as water or soil and rarely cause disease. Only a few of them cause disease in mammals and are known as intracellular pathogens. M. tuberculosis, M. bovis, M. africanum, and M. microti are collectively called M. tuberculosis complex. All the members of M. tuberculosis complex are pathogenic. M. tuberculosis and M. africanum are pathogenic for humans and M. bovis is usually pathogenic for animals but also can be transmitted to humans. M. microti is a pathogen of voles but is avirulent in humans and mice. The cell wall structure of mycobacteria is very different from other fast-growing bacteria. The complex mycobacterial cell wall is structured differently, consisting of unusual amounts of lipid moieties i.e. 11 peptidoglycolipids (mycosides), cord factors and sulpholipids. M. tuberculosis complex organisms can be stained with carbolfuchsin and appeared to be rod-like shape when visualized under a microscope. Using acid or alcohol for decolourisation, mycobacteria could retain the colour and hence, they are also called ‘acid-fast bacilli’. Host-pathogen interactions Airways infection with M. tuberculosis leads to sampling of organisms by the primary host cells such as alveolar macrophages. As a consequence, bacteria become arrested inside of a phagosome and later being delivered to the lysosome by phago-lysosome fusion in order to be killed by the host cell. M. tuberculosis could interfere with the host killing machineries and establish their fate inside the host either following a state of ‘dormancy’ or causing active disease (7). Mycobacterial survival strategies There are several strategies that have been described in relation with mycobacterial survival within a host. Intrinsically, mycobacterium could withstand the host killing machineries due to the unique features of the mycobacterial cell wall. Depletion of the cell wall components reduces bacterial virulence activity, suggesting the importance of the cell wall integrity towards survival of mycobacteria inside the host (8). Formation of phosphatidylinositol 3-phosphate (PI3P) is important in regulating a normal host cell trafficking event. PI3P functions as a docking site for proteins, which is required for the maturation of phagosomes into lysosomes. (9, 10). Mycobacteria could inhibit the process of accumulation of PI3P on phagosomal membrane and thereby block phagosome-lysosome fusion. It was postulated that M. tuberculosis toxin lipoarabinomannan (LAM) could block the cytosolic increase of Ca2+ and inhibit a novel Ca2+/Calmodulin-PI3K hVPS34 cascade, which is essential for the synthesis of PI3P on phagosomes (11). As shown in Fig. 1, once inside the phagosome, M. tuberculosis secretes virulence factors such as SapM (an eukaryotic-like acid phosphatase) and serine/threonine kinase PknG to inhibit phagolysosome fusion (7). It is thought that SapM may hydrolyse PI3P and PknG may phosphorylate host molecules, thereby preventing the formation of phago-lysosome formation (7). Host tryptophan aspartate containing coat protein (TACO), also called P57, recruitment enhanced when phagosomes harbour live mycobacteria but the TACO protein levels dropped when phagosomes contain killed bacteria, suggesting that TACO is another component that 12 interferes with the lysosomal delivery (12, 13). Active retention of TACO leads to the activation of calcium-dependent phosphates calcineurin, which is being considered as a critical factor for blocking lysosomal delivery although the precise mechanism is still unknown. Fig. 1: Macrophage antimicrobial activity and escape mechanisms for the survival of M. tuberculosis. Once inside the host cell, M. tuberculosis secretes SamP and PknG to be able to inhibit the phagosome-lysosome fusion. Accumulation of TACO protein around the phagosome interferes with the phagosome-lysosome fusion. LAMP1 and V-ATPase are lysosomal proteins. The Toll-like receptor (TLR) signaling can modulate phagosome-lysomal fusion through p38MAPK pathway and also TLR signaling can increase the production of antimicrobial peptide LL-37 via the upregulation of vitamin D receptor. (Cell Host Microbe. 2008,3:399-407, reprinted with permission from Elsevier Inc.) Adaptation of macrophages to infection In most infected individuals, who do not develop active TB, a delicate balance is established between the host immune response and the M. tuberculosis virulence, which is termed ‘granuloma formation’ (14, 15). The structure of granulomas is a cluster of M. tuberculosis living inside macrophages surrounded by other cells. Within this granuloma, mycobacteria are kept in check so that they are not able to cause disease. The exact biology of granuloma formation is still not completely understood, however, it is believed that mycobacteria in such a condition can be actively dividing or be silent even within a same individual (16, 17). Under appropriate activation, macrophages process antigens and present them to T lymphocytes. Activated T cells produce cytokines and chemokines and that result in further activation of macrophages or an influx of other immune cells to the site of granuloma. A discontinuation of 13 this check-in process due to any suboptimal host response contributes to M. tuberculosis release and dissemination to other organs, which can be ended up with active TB (18). Susceptibility to infection Many people exposed to M. tuberculosis bacilli do not contract infection. Also, the infection with M. tuberculosis in humans poses only a 10% lifetime risk of developing active TB. Since the vast majority of the infected people do not develop active TB, this perhaps indicates that the large interindividual variability in the induction of immune responses and/or the genetic predisposition are probably associated in part with the variable outcome of infection. Previous studies have gained insights into this complex phenomenon and unveiled many environmental factors such as first-contact epidemics that increase the susceptibility (19), poor economy, malnutrition, stress, overcrowding, which all enhance the susceptibility to TB in humans (20). Apart from all those factors, there have been many studies performed in humans (21, 22) or animals (23-26) revealing that host genetic factors play a significant role in the outcome of the M. tuberculosis infection. Searching for genetic components of susceptibility to M. tuberculosis infection in humans has been a difficult task. Recently, forward genetic approaches in mice and humans have been used to explain the molecular basis for predisposition to mycobacterial diseases (27). Genome wide screening of both resistant and susceptible mouse strains with M. tuberculosis infection identified 18 genes in resistant and 120 genes in susceptible strains that are regulated selectively (28). Further characterization of some of those genes revealed that macrophages from susceptible strains induced more inflammatory responses and caused tissue damage (28). A similar study has also found a group of genes responsible for tissue fibrosis showing very high levels of changes in gene transcripts in the susceptible mouse strains (29). Structure of the lung pathology has been found to be different in the resistant mouse strains compared to the susceptible strains, where a large aggregate of lymphocytes was found close to the granulomas. Only a few lymphocytes with other immune cells were present surrounding the granulomas in the susceptible strains suggesting that a generalized defect in the presence of lymphocytes might contribute to the susceptibility of infection (30). Thus far, a vast majority of the work in humans focused on some of the candidate genes and their association with TB susceptibility. The natural resistance-associated macrophage protein gene 1 (NRAMP1), a homologue of a gene (Nramp 1) on mouse chromosome 1 has been reported to be very critical in controlling TB. Polymorphisms in NRAMP1 have been demonstrated to be a risk factor for adult (31) and paediatric TB (32). A 14 metaanalysis of studies with NRAMP1 polymorphisms revealed that polymorphisms were associated with pulmonary TB in African and Asian populations but not in populations of European descent (33). Conflicting results have been obtained from recent studies on SP110 variants (a nuclear body protein) and TB susceptibility in patients from West Africa (27). An association between MHC genes, for example, alleles encoding an aspartic acid at codon 57 of HLA-DQ β-chain and pulmonary TB was reported. HLA-DQ β57-Asp showed reduced ability to bind with a peptide from early secreted antigenic target 6 (ESAT-6) protein (34). Polymorphisms in the gene encoding DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3 (ICAMP-3)-grabbing non-integrin) have also been reported to be associated with the adult pulmonary TB in a South African population (35). The susceptibility to mycobacterial infection can also be assessed by ‘genetic mutation’- that targets one or more genes of interest and results in malfunction of proteins after expression. In fact, genetic mutations have been recorded in many infected individuals, which even can transmit from person to person followed by Mendelian inheritance. Genes that were identified to be responsible for the susceptibility to mycobacterial infection centre around interleukin 12 (IL-12) and interferon gamma (IFN-γ) axis include IFN-γR1 and IFNγR2, two chains of IFN-γ receptor; IL-12β, encoding the p40 subunit of IL-12; IL-12 βR1, the β1 subunit of the IL-12 receptor; and signal transducer and activator of transcription-1 (STAT-1) (36). Mutations in IFN-γR2 and IL-12p40 have been found to be associated with the susceptibility to M. tuberculosis infection but most of the others are demonstrated in relation with M. bovis or environmental mycobacterial infection (36). Immunology of TB In normal circumstances, exposure to foreign molecules turns on a series of defence reactions in the body in order to develop an effective protection. The immune system is classified into two distinct branches based on the nature of their responses; one called innate immune system, non-specific and another called adaptive immune system, specific. M. tuberculosis infection can induce both innate and adaptive immune responses in humans as well as in experimental animal models. Since the 90% of the exposed individuals do not develop active TB, this observation suggests that the immune system plays pivotal roles in controlling disease. 15 Innate immunity The innate immunity is the first-line of defence against pathogens that comes quick in a nonspecific manner and has no recall power. The innate immune system is activated upon recognition of microbial components by a number of receptors on host cells. For the detection of M. tuberculosis, macrophages use several receptors, which include mannose receptor (MR), complement receptor (CR), class A scavenger receptor, dectin 1 (C-type lectin), DCSIGN, TLRs and the nucleotide oligomerization domain (NOD)-like receptors (Fig. 2). Mycobacterial interactions with innate receptors Mannose receptors interact with the mannose-capped lipoarabinomannan (ManLAM) on M. tuberculosis that facilitate attachment and internalization of bacilli by macrophages. M. tuberculosis ManLAM blocks phagosome-lysosome fusion and thereby enhances survival of M. tuberculosis in human macrophages. Complement receptors promote phagocytosis of M. tuberculosis by macrophages. Activation of the alternative complement pathway by M. tuberculosis promotes opsonisation mediated by the complement components C3b and iC3b (37). This allows the recognition of bacilli by CR1, CR3 and CR4. In the absence of CR1, patients with TB disease have increased levels of immune complexes that enhance the severity of the disease (38). However, studies in murine models of TB showed that CR3 deficiency did not have any significant effect on phagocytosis or alteration of the course of the disease (39). Alternatively, in the absence of CR3, M. tuberculosis could gain entry into the host cells by other phagocytic receptors and establish infection (39). DC-SIGN is a C-type lectin, initially found on the dendritic cells and later it was also found on alveolar macrophages (40). DC-SIGN interacts with LAM, lipomannan, and arabinomannan antigens of M. tuberculosis (41-43). DC-SIGN, one particular pattern recognition receptor (PRR), can induce immune responses by modulating TLR-induced activation at the level of the transcription factor NF-kappaB (44). It has been described that DC-SIGN not only could interact with M. tuberculosis but also with other pathogens e.g. M. leprae, Candida albicans, measles virus, and HIV-1 and activate the NF-kappaB signalling pathway. Upon interaction with M. tuberculosis DC-SIGN on DC triggers a cascade of signalling pathway including activation of serine and threonine kinase Raf-1, which subsequently promotes acetylation of the NF-kappaB subunit p65 provided that NF-kappaB is 16 also activated by TLR-induced signalling (44). Acetylation of NF-kappaB leads to increased and sustained levels of IL-10 to enhance anti-inflammatory responses. Fig. 2: Innate receptors and ligands for recognition of M. tuberculosis (Immunol Rev. 2007 ;219:167-86, reprinted with permission from John Wiley & Sons Inc.). TLRs are very important in the course of interaction with mycobacterial components. TLRs are a type of PRRs that interact with microbes/pathogen associated molecular patterns and that subsequently help in phagocytosis and induction of immune responses. Ten members of TLR family have been identified in humans (45). Different components of microbes are recognized by different TLRs, for example, lipopeptides interact with TLR2, which forms dimer with TLR1 or TLR6; lipopolysaccharide is recognized by TLR4; flagellin by TLR5 and CpG DNA by TLR9. In TB, a mycobacterial component particularly lipoprotein is recognized by TLR2 and the role of TLR2 has been described as central in many cases (46, 47). TLR1/6, 9 and also TLR4 have been found to interact with mycobacterial components (48, 49). The 19kDa lipoprotein, a secreted antigen of M. tuberculosis, soluble TB factor, protein-free 17 short-term culture filtrate of M. tuberculosis signal through TLR2 and similarly, mycobacterial cell wall components such as LAM, lipomannan, phosphatidyl-myo-inositol (PIM) interact with TLR2 (49). CD14, a coreceptor of TLR4, is present on the macrophages, dendritic cells, or neutrophils. CD14 has no cytoplasmic signalling domain. CD14 has been found to interact with mycobacterial AraLAM and activated cells in a TLR2-dependent manner (50). Bonemarrow derived macrophages from TLR2-/- and TLR4-/- infected with live BCG have confirmed the involvement of TLR2 signalling and to a lesser extent TLR4 signalling for the production of tumor necrosis factor (TNF) and IL-12 (51). TLR signalling is also very important in the vitamin D activation pathway. Vitamin D could induce antimicrobial activity against M. tuberculosis as has been suggested by Rook et al in 1986 (52). Recently, it has been shown that activation of TLR2/1 augments vitamin D receptor and 25-hydroxyvitaminD3-1α-hydroxylase, which are important for the conversion of the pro-form of vitamin D to an active form. The activation of vitamin D pathway in a TLR-dependent manner leads to the synthesis of antimicrobial peptides for example, cathelicidin in humans (53). Low levels of vitamin D and the risk of developing TB have been demonstrated in human studies (54). The nucleotide oligomerization domain (NOD)-like receptor (NLR) also interacts with the mycobacterial components. NLRs are cytoplasmic proteins that belong to a TLR-related protein family, which have a C-terminal leucine-rich domain, central nucleotide-binding domain and N-terminal protein-binding domain (36). There are two types of NLRs; NOD1 and NOD2 that could interact with peptidoglycans, muramyl dipeptides and diaminopimelatecontaining N-acetylglucoseamine-N-acetyl muramic acid tripeptide. Mycobacterial muramyl dipeptide interacts with NOD2 and induces cytokine production, this synergizes with the 19kDa-mediated activation of TLR2 and cytokine production (55). Ferwerda et al suggested that defective expression of NOD2 results in 80% reduction of the cytokine production by mononuclear cells after stimulation with M. tuberculosis and the lack of function of either of TLR2 or NOD2 causes the loss of synergism (55). Therefore, it has been proposed that NOD2 pathway is an independent and nonredundant in the recognition of M. tuberculosis (55). Activation of NLRs causes downstream signalling via two pathways: activation of caspase-1 and the NF-κB pathway, which ends up with the production of α-4 defensins or cryptdins. α-4 18 defensins or cryptdins have bactericidal activity against M. tuberculosis (56). Despite the fact that polymorphism of NOD is linked to several inflammatory diseases, and NOD deficiency has in vitro effects on M. tuberculosis recognition, animals are not susceptible to M. tuberculosis infection due to the lack of NOD2 (57). Innate cells Macrophages Macrophages are known as the primary habitat for mycobacteria. Monocytes become activated and differentiated into macrophages. Macrophages are one of the most important professional antigen-presenting cells (APCs), which can phagocytose, process and present antigens to the T lymphocytes in an association with major histocompatibility complex (MHC) molecules. One hundred years ago Metchnikoff, who received the Nobel Prize in 1908, discovered that macrophages are able to phagocytose and have a potential role in the host-defense mechanism. It is proposed that alveolar macrophages engulf mycobacteria after entering the host via the nasal route and subsequent activation of macrophages attracts more macrophages from the bloodstream. Macrophages harbour many receptors on their cell surface and inside which are important for the interaction with M. tuberculosis. Depending on the type of receptor-ligand interaction, macrophages generate different types of immune responses. In vitro experiments have shown that pretreatment of bacilli with immune sera enhances attachment and phagocytosis of bacilli and also accelerates phagosome-lysosome fusion (58). In contrast, CR3-mediated nonopsonic internalization of pathogenic mycobacteria (M. kansasii) does not trigger the formation of oxygen intermediates (59) and blocks the maturation of phagosomes, and prevents phagosome-lysosome fusion (60). TLRs mediated sensitization of macrophages promotes activation of the NFκB signaling pathway and enhances IL-12, TNF and NO synthesis. These mediators stimulate the microbicidal pathway to kill the ingested mycobacteria. Dendritic cells DCs are considered as the frontline sentinels of the body defence system. Like the macrophages, DCs are also known as professional APCs as they can engulf, process and present antigens on the surface of other cells with the help of MHC molecules. Recognition of mycobacterial components by DCs occurs by interaction with C-type lectin receptors, DCSIGN, and TLR receptors. Interestingly, phagocytosis of M. tuberculosis by interacting ManLAM with DC-SIGN is not associated with the inhibition of phago-lysosome fusion as 19 suggested by Kang et al, which is in contrast to the observation when macrophages engulf mycobacteria by engaging the MR with M. tuberculosis ManLAM (61). TLR9 in DCs could recognize mycobacterial DNA released from the ingested bacteria and promote IL-12 secretion, which is not the case for macrophages infected with M. tuberculosis (62). M. tuberculosis infection causes rapid remodelling at the IL-12p40 promoter and thereby increases IL-12p40 transcription only in the DCs in a TLR9 dependent manner. However, in the macrophages this occurs in a TLR2-dependent manner (62). The critical role of DCs in M. tuberculosis infection is probably the initiation of the immune responses (63). DCs sample mycobacteria/secreted antigens in the lungs and carry them to the regional draining lymph nodes and that is essential for the initiation of immune responses (64, 65). However, it is not proven whether the activation of T cells in the draining lymph node occurred by the direct interaction with the lung-derived bacteria-infected DCs. Epithelial cells Epithelial cells form a single layer lining over the alveolar lumen and are thought to be the first cells that M. tuberculosis adheres to during invasion into the host tissues. There are two major types of cells that are important for the maintenance of alveolar epithelium; a thin, squamous, type I cells that cover 95% of the epithelium and cuboidal type II cells. Earlier studies have shown that mycobacteria could infect and multiply inside the type II alveolar cells (66). Identification and characterization of heparin-binding hemagglutinin adhesin (HBHA) from M. tuberculosis and M. bovis have revealed that mycobacteria use HBHA to adhere on the surface of the epithelial cells by interaction with sulphated glycoconjugates (67). It remains to be unveiled if epithelial cells have any role in the induction of immune responses. Saiga et al demonstrated that Lipocalin 2 (Lcn2), also known as neutrophil gelatinase-associated lipocalin, produced by epithelial cells and macrophages during the early phase of mycobacterial respiratory infection, is important for the host defence against M. tuberculosis (68). Lcn2-deficient mice are susceptible to intratracheal route of M. tuberculosis infection. Lcn2 seize iron and therefore inhibits the mycobacterial growth in the epithelial cells but not in alveolar macrophages (68). Debbabi et al have demonstrated in murine models that type II alveolar cells become activated upon infection with M. tuberculosis and express cell surface class II MHC, CD54, and CD95 molecules (69). Also, type II cells can present mycobacterial antigens to immune CD4 T cells isolated from mice infected with M. tuberculosis (69). 20 Neutrophils Neutrophils are the most abundant white blood cells belong to the polymorphonuclear family. Neutrophils are circulating in the bloodstream but in response to acute infection migrate to the site of infection and show microbicidal activity (70). Neutrophils are the reservoirs of granules with high concentration of antimicrobial activity. Two major types of neutrophil granules are characterized: primary granules that contain α-defensins, myeloperoxidase, and serprocidins, and secondary granules that contain lactoferrin, cathelicidin, and neutrophil gelatinase-associated lipocalin. Upon inflammatory responses, neutrophils undergo apoptosis and macrophages phagocytose the apoptotic neutrophils in order to clear toxic substances from the body. Tan et al have shown that M. tuberculosis infected macrophages can readily phagocytose apoptotic neutophil and acquire antimicobacterial activity (71). Experimental animal models of TB have shown that neutophils are found in the lungs at early times of infection as well as some days after initial infection (72, 73). Defective neutrophil functions or depletion of neutrophil activity exacerbate myobacterial growth in different organs and reduce IFN-γ levels and nitric oxide synthase activity (72), suggesting the protective role of neutrophils in the host defence mechanism. Recently, it has been described that interaction between neutrophils and DCs could occur through Mac-1 expressed on neutrophil and DCSIGN expressed on DC, which promotes DC maturation by TNF secretion (74, 75). Apart from being involved in immune protection, accumulation of neutrophils can be harmful for the host due to pathology because susceptible animals with M. tuberculosis infection have larger and longer accumulation of neutrophils in TB lesions (76). Natural killer (NK) cells NK cells are cytotoxic lymphocytes, which play an important role in the innate immune system. NK cells are activated by cytokines and once activated bind to the Fc portion of antibodies and perform antibody-mediated cellular cytotoxicity in order to lyse infected cells. Regulation of NK-cell activity is maintained by so called ‘activating and inhibitory receptors’ that help to differentiate between the infected and normal cells. Studies by Junqueira-Kipnis et al have suggested that NK cells have only minimal role in protection against M. tuberculosis (77). NK cells are increased in numbers during the early stage of infection, however, depletion of NK cell activity does not show significant effect on pulmonary bacterial load (77). However, it has been found that human NK cells could contribute to immune defences against M. tuberculosis by producing IL-22 cytokine. IL-22 cytokine producing human NK cells promote phagosome-lysosome fusion and result in the inhibition 21 of mycobcaterial growth (78). Feng et al have shown that NK cell-driven IFN-γ production functions in a T-cell independent way in T-cell deficient (RAG-/-) mice infected with M. tuberculosis (79), suggesting a possible significant role of NK-cell mediated immunity in HIV-infected individuals. Adaptive immunity The adaptive immunity is highly specific to a particular antigen previously encountered by the immune system. Two major components are involved in maintaining this host adaptive immunity: one is called cell-mediated which includes T cell activation and effector mechanisms and another is humoral immunity where B cells and antibodies are involved. M. tuberculosis infection in humans is intracellular and thereby cell-mediated immune responses are regarded to be very important in host protection. Humoral/antibody-mediated immune responses may also contribute to the immune resistance to TB (80, 81). Cell mediated immunity to mycobacteria CD4 T cells CD4 T cells, also known as helper T cells, play a significant role in directing/regulating the adaptive immune system. Initiation of T-cell mediated immune responses begins upon activation of T cells by the innate immune cells e.g. APCs. Without the cooperation from the innate cells T cells remain naive. The importance of CD4 T cells in protection against TB is tremendous because HIV-mediated decrease of CD4 T cell number results in progressive primary infection, reactivation of latent infection and debilitate the condition of patients with TB disease (82-85). In mouse models of TB, the requirement of CD4 T-cell mediated immune responses has also been demonstrated by using CD4 T cell knockout animals or by passive transfer of CD4 T cells (86, 87). Passive transfer of CD4 T cells is associated with an early protection against M. tuberculosis (88). A defective CD4 T-cell function or MHC II molecule increases the susceptibility to M. tuberculosis infection, revealing the central role of CD4 T cell in protection (89). Cytokines produced by CD4 T cells are of critical importance for the regulation of Th1 or Th2 type of immune responses. Th1 cells secreting IL-2 and IFN-γ, are associated with cell-mediated immunity, whereas Th2 cells that produce typically IL-4, IL-5, IL-10 and IL-13 are responsible for the regulation of humoral immunity. Upon antigen presentation, CD4 T cells become activated and produce the key cytokine IFN-γ. Macrophages are activated by IFN-γ and produce antibacterial components i.e. reactive oxygen and reactive nitrogen intermediates in order to kill the bacteria, which is 22 the major effector mechanism of cell-mediated immunity against TB. CD4 T cells have been reported to express cytotoxic activity (90). CD4 cytotoxic T lymphocytes (CTL) preferentially lyse their targets via Fas-Fas ligand interaction, whereas the major cytotoxic effect of CD8 CTL is mediated by perforin and granzymes. Although some CD4 CTL may kill the targets by perforin and granzymes, this pathway is of limited significance (90). CD8 T cells CD8 T cells or cytotoxic T cells recognize peptide antigens in the context of MHC class I molecules. Although it is believed that CD8 T cells are less important than CD4 T cells in protection against TB, defective function of CD8 T cells due the loss of function of β2 microglobulin mice succumb to M. tuberculosis infection (91). M. tuberculosis mediated activation of CD8 T cells enhances IFN-γ, granulysin, Fas-L, and perforin synthesis, which act through different mechanisms on infected cells and kill the bacteria (92). In human TB, CD8 CTL have been found to act directly on the infected cells and kill the mycobacterial pathogen. It has been suggested that granulysin alters the integrity of bacterial cell and in combination with perforin, reduces the viability of M. tuberculosis (93). Other evidence showed that a subset of CD8 T cells could express CCL5 chemokine together with perforin and granulysin and the presence of CCL5 attracts M. tuberculosis infected cells which enhances the clearance of M. tuberculosis from the host (94). Although perforin and granzyme have direct mycobactericidal activity, gene knockout experiments with perforin and granzyme have shown that there is no discernible influence of the lack of expression of perforin or granzymes on the course of M. tuberculosis infection and pathology (95) tested in mice. This suggests that other mechanism of protection offered by CD8 T cell probably exists and that it might be cytokine dependent. A passive transfer of CD8 T cells from the control mice to the infected mice improved the course of M. tuberculosis infection and it failed if the cells were taken from IFN-γ gene knockout mice, indicating the importance of IFN-γ cytokine produced by CD8 T cells in protection (96). M. tuberculosis antigen processing and presentation to CD8 T cells are likely to be operated by three different mechanisms (called cross-processing) other than the classical MHC class I antigen presentation (97). First, exogenous antigens can be taken up by the APCs for MHC class I antigen processing and presentation; second, M. tuberculosis infected cells may produce exosomes containing mycobacterial antigens, which can be presented by bystander APCs for MHC class I cross processing; third, apoptosis of M. tuberculosis infected 23 cells releases apoptotic vesicles containing the mycobacterial antigens, which are taken up by bystander APCs for MHC class I cross processing. In addition to the classical MHC class I A, B, C mediated recognition, studies in humans with M. tuberculosis infection explained that recognition of mycobacterial peptide antigens by CD8 T cells could be possible via a novel nonpolymorphic MHC class Ib antigenpresenting pathway (98). Lewinsohn et al (99) assessed the frequency of classically and nonclassically restricted CD8 T cell clones among 96 M. tuberculosis positive CD8 T cell clones and observed that the classically restricted CD8 T cell clones are very few (4%) compared to the non-classically restricted CD8 T cell clones (96%) suggesting that the classically restricted CD8 T cells comprise only a small part of the total M. tuberculosis specific CD8 T cells. Th17 cells More than 20 years ago, Mosmann and Coffman (100) described two subsets of Th cells (Th1 and Th2) based on their distinct patterns of cytokine expression as explained above. More recently, a new subset of Th cells named Th17 has been characterized primarily based on their ability to produce IL-17 cytokine (101). IL-17 cytokine was described in mice, rats and humans in the mid-1990s and proposed that IL-17 could play an important role in tissue inflammation. New experimental findings have further shown that Th17 cells are important not only for the resistance to fungal infection and for the mucosal immunity but also can be instrumental for the development of organ-specific autoimmunity (102). IL-23 cytokine has been proposed to be important for the Th17 responses (101). Activation of human DCs through the combination of ligands for TLR2 and NOD2 is required for the production of IL23 which is in contrast to the IL-12 production where additional activation by IFN-γ-priming and/or costimulation with TLR7/8 ligand (R848) are necessary (101). IL-23, which is required for the generation of Th17 responses, may participate in the vaccine-induced protection against M. tuberculosis infection. Experimental murine models of TB have shown that IL-23 production accelerates IFN-γ producing CD4 T cell responses and the establisment of an IL-17-producing CD4 T cell population in the lungs. It has been shown that vaccination with I-Ab-restricted ESAT-6 [1–20 amino acids] epitope induces IL-17producing CD4 T cell population in the lungs and upon challenge with M. tuberculosis, Th17 cells express chemokines CXCL9, CXCL10 and CXCL11, which are required for the recruitment of IFN-γ producing CD4 T cells in the lungs (103). 24 Gamma delta (γδ) T cells γδ T cells are a group of cells that contain a distinct T cell receptor (TCR) molecule, called γδ TCR, which is composed of γ chain and δ chain. Unlike αβ T cells that recognize processed antigens in an association with the MHC molecules, γδ T cells recognize natural and synthetic non-peptide antigens (104-106). Little is known about the conditions for γδ T-cell activation but their role in host immune responses could be involved during the early stage of infection by collaborating between the innate and adaptive immune system (107). γδ T cells function independently in the airway, do not need help from αβ T cells (108). In humans with mycobacterial infection, the frequency of mycobacterial antigen specific γδ T cells was found to be increased and their role in IFN-γ production and cytotoxic activity has been reported previously (109, 110). γδ T cells do express Fas-FasL and perforin to a similar extent as CD4 and CD8 T cells express in both patients with M. tuberculosis infection and healthy controls (111). Studies in mouse models of TB have suggested that γδ T cells might have antiinflammatory activity against M. tuberculosis. Depletion/defective functionality of γδ T cells accelerates inflammatory damage in the lungs with M. tuberculosis infection (112). γδ T cells express IL-23 receptor and thus can produce IL-17 in response to IL-1β and IL-23 cytokines (113), which might promote cell migration into the site of infection (114). Apart from being cytokine producers, γδ T cells are also able to function as professional APCs and give sufficient costimulatory signals to αβ T cells for the induction of proliferation and differentiation (115). Thus, γδ T cells are called as alternative type of professional APCs (116) and novel initiators for adaptive immunity (117). Regulatory T cells (Treg) Treg cells are primarily known as suppressive T cells, function to control immune responses against self antigens. Treg cells represent 5-10% of CD4 T cells and are characterized by a specific transcription factor, forkhead box p3 (Foxp3). A large portion of the Treg cells expresses CD25 marker and thus the phenotype of Treg cells is denominated as CD4+CD25+ cells. It has become clear that the Treg cells not only control self-immune responses but also respond to foreign antigens (118). Given that a delicate balance may exist between the virulence of M. tuberculosis and the host responses following infection, it has become important to assess the functional capacity of regulatory cells in M. tuberculosis infection. It has been reported by Ribeiro25 Rodrigues et al that the frequency of CD4+CD25+ and CD4+CD25high T cells was increased in patients with active TB and remained elevated at completion of six months of therapy (119). The cytokines IFN-γ and IL-2 are suppressed in patients with active TB but IL-10 and TGFβ1 levels increase, therefore overproduction of IL-10 and TGF-β1 has been implicated for the decreased T-cell function in TB (119). Although following antituberculosis treatment IL-10 and TGF-β1 levels drop, T-cell responses remain suppressed suggesting that additional mechanisms might control T-cell responses during active TB. TGF-β1 has been shown to act on the conversion of CD4 T cells into the Treg cells and also TGF-β1 is required for survival, retention and function of the Treg cells (120). However, there is no clear indication that CD25+ T cells produce IL-10 and TGF-β1. The role of Treg cells in the suppression of IFN-γ production has been established by cell depletion experiments where the depletion of CD25+ T cells increased IFN-γ production by CD4 T cells compared to the undepleted cells (119). Moreover, increased levels of Treg cells have been observed in patients with extrapulmonary TB (121). Extrapulmonary TB manifests due to the failure of the Th1-type of immune responses and that causes mycobacteria to migrate to another place. A balanced immune response is needed for the control of infection or extensive pathology. The majority of the infected individuals who do not develop active TB are believed to be fine-tuned with the immune responses. It is considered that the Treg cells down-regulate the immune responses following eradication of pathogens to avoid the development of pathology, however, this may lead to the establishment of chronic infection with M. tuberculosis. Humoral immunity B cells are able to perform the role of APCs and are recognized as the key players for the induction of humoral or antibody-mediated immune responses against antigens. B-cell activation can take place in two ways: T-cell dependent or T-cell independent manner. In the T-dependent activation, antigen-presenting macrophages activate helper T cells with matching receptors and promote T cell and B cell interaction. This process induces memory B-cell formation and also triggers proliferation and differentiation into antibody forming plasma cells. In the T-independent activation, B cells can be activated by thymus independent antigens. For example, many repeating carbohydrate epitopes that are present on bacterial surfaces can cause cross-linking of antibodies on the surface of B cells and result in activation of the B cells. Circulating antibodies or cell bound antibodies function in the complement 26 activation, neutralization of toxins, opsonisation of pathogens and finally enhance the elimination of pathogens by phagocytosis. Since M. tuberculosis is an intracellular pathogen, the role of antibodies in protection against TB is generally regarded as nonprotective. However, antibodies might be very important in neutralization and prevention of invasion of pathogens especially at the mucosal surface. TB is primarily a respiratory mucosal disease, therefore, antituberculous antibody research gained renewed interest and it has already been shown that antibody could provide protection against TB (122, 123). In the absence of B cells, mice had exacerbated immunopathology and a large number of neutrophil accumulation which coincides with increased bacterial burden (124, 125). Adoptive transfer of B cells to the B-cell deficient mice could reduce the pathology and increase serum antibody levels, suggesting an immunoglobulin mediated ‘endocrine’ regulation (125). In the BCG-infected experimental models, mycobacteria-specific antibodies have been shown to increase uptake and killing of BCG bacteria by neutrophils and macrophages (126). Antimycobacterial antibodies have an effect on the cell-mediated immunity as demonstrated by de Valliere et al (126). They observed that in vitro antibody-coated BCG bacteria were taken up by DCs more efficiently and could stimulate CD4 and CD8 T cells for IFN-γ production. Rodríguez et al have shown that IgA-deficient mice were more susceptible to M. bovis BCG infection (127). Upon immunization IgA-deficient mice were unable to produce IgA and also cytokine responses were reduced compared to the wild-type control mice (127). Among several mycobacterial antigens, mycobacterial arabinomannan, HBHA and 16kDa α-crystallin have been reported to induce protective antibody responses (122, 123, 128-130). It remains to be unveiled how antibodies could contribute to the host protection. Maglione and Chan have proposed a possible mode of action of antibodies together with Fcγ receptors (Fig. 3) (131). Upon interaction with stimulatory Fcγ receptors on macrophages, Th1 responses can be enhanced due to the increased production of IFN-γ. Because upon infection with M. tuberculosis mice deficient in inhibitory FcγRIIB receptors have been found to develop increased Th1 responses evidenced by the increased IFN-γ producing CD4 T cells (132). On the other hand, B cells have a significant impact on IL-10 cytokine production since an increased level of IL-10 production has been shown to be associated with B-cell deficiency (133) and defective function of Fcγ-stimulatory receptor (125, 134). Thus, it is assumed that the excessive levels of IL-10 might be linked with immunopathology during an infection with M. tuberculosis (125). 27 Fig. 3: Proposed mechanism of antibody-mediated modulation of immune responses in TB (Eur J Immunol. 2009;39:676-86, reprinted with permission from John Wiley & Sons Inc.). B-cell function seems to be varied during the course of M. tuberculosis infection as suggested by Maglione and Chan (131). B cells contribute to optimal granulomatous response during the acute condition of infection and help in the containment of bacilli. During the latent infection, it is thought that B cells promote local host immune responses against M. tuberculosis and that might restrict reactivation of infection. Cytokines and chemokines Cytokines and chemokines are essential components of the immune system. Cytokines and chemokines contribute to the formation of a cell network by delivering signals to the cells and helping them to the induction of effector functions. Cytokines Cytokines such as IL-12, IFN-γ, IL-2 and TNF play an essential role in the maintenance of protective immunity against TB. Experiments in animal models with the loss of function of a cytokine gene or an inhibition of cytokine function by administration of a specific antibody revealed the need of cytokine function for the host protective immunity (135-138). Similarly, a defective cytokine receptor function is also associated with the lack of protective host responses against TB (139, 140). IL-12 is produced by macrophages or DCs upon phagocytosis of M. tuberculosis. IL-12-regulated IFN-γ production by T cells or NK cells is a key pathway for the control of M. tuberculosis infection (141, 142). IL-12p40 but not IL28 12p35 is essential for the regulation of IFN-γ production since gene knockout of the IL-12p40 subunit causes reduced IFN-γ production and mice succumb to infection (142). IL-12p40 is critically involved in the migration of DCs to the regional lymph nodes to activate T cells (143). IFN-γ activates macrophages, CD4 and CD8 T cells for the induction of effector functions in order to kill the pathogen. Activation of macrophages starts the synthesis of nitric oxide (NO), and related nitrogen intermediates (RNIs) upon the action of inducible nitric oxide synthase (iNOS), which facilitate the killing process. In murine models, RNIs mediated antimycobacterial effects are well documented (144, 145), however, it is still uncertain how much of this is relevant to human TB. Upon antigen presentation by macrophages, activated T cells produce IL-2, which causes expansion of the antigen specific T cells. TNF is an important cytokine in the control of TB. TNF regulates the effective granuloma formation and thereby prevents bacterial dissemination. TNF influences the expression of adhesion molecules and also chemokines that attract immune cells to the infected tissues (146). In absence of TNF, effective granuloma formation is impeded and bacterial growth is rapidly increased, which reduced the survival time of the mice (147, 148). TNF functions by forming a trimer with its receptor - either with TNF receptor 1 (TNFR1, 55 kDa) or TNFR2 (75 kDa), present on the cell membrane and expressed by almost all nucleated cells. TNFRs belong to the TNF receptor superfamily. The extracellular domain of both receptors is cleaved by metalloproteases and then the soluble form (sTNFR) binds to TNF and neutralizes TNF-mediated activities. It has been described that the complete neutralization of TNF activity by sTNFR prevents the induction of cell-mediated immunity in mice and therefore mice succumb to BCG infection (149). Lower sTNFR levels result in higher bactericidal activity of macrophages by enhancing iNOS activity (149). IL-4, one of the cytokines that drives the immune system to induce Th2 type response, is considered as the harmful candidate for the host protective immunity against TB. IL-4 has been implicated with the poor efficacy of BCG vaccine in the developing world where a preexisting enhanced IL-4 immunity in people with helminth infection is characterized (150). Elevated levels of IL-4 have been observed in patients with pulmonary TB (151, 152). The increased IL-4 levels seem to be correlated with serum IgE concentration and with the extent of cavitation (151, 152). In mouse models of TB, IL-4 has been shown to downregulate iNOS activity (153). IL-4 delta 2 (IL-4δ2), a splice variant and inhibitor of IL-4 has been described recently and found that both are increased in active TB and only IL-4δ2 is elevated in individuals with latent infection (154, 155). 29 Chemokines Chemokines, also known as chemotactic cytokines, are small proteins, 8-10 kDa in size. The main function of chemokines is to call immune cells to a specific place (infected site) in order to fight against the pathogens. Changes in the expression pattern of chemokine receptors (CCR) determine whether the cells will migrate to another place. Chemokine receptors belong to four different groups; CXC, CC, CX3C and XC that correspond to the 4 distinct subfamilies of chemokines they bind. Dendritic cells upon antigen stimulation downregulate CCR5 and CCR1 expression and increase CXCR4 and CCR7 expression which promote migration of DCs to regional lymph nodes in order to activate T cells (156). M. tuberculosis infection leads to the synthesis of chemokines by cells as early as 2 h postinfection. Upon M. tuberculosis infection, human macrophages produce a range of CC chemokines CCL2, CCL3, CCL4 and CCL5 (alternate names MCP1, MIP1α, MIP1β and RANTES) (157). Higher levels of MCP1, RANTES, IL-8 but not MIP1α were detected in bronchoalveolar lavage of TB patients (157). Increased levels of CCL5 correlate with the higher number of CD4 T cell in bronchoalveolar lavage of TB patients (158). Studies in murine models with CCR2 knockout gene have revealed that cell migration is substantially delayed as well as IFN-γ and NO production upon low dose infection with M. tuberculosis (159). However, there was no difference observed in the bacterial growth between the knockout and wild-type control mice. Granuloma formation was not hampered in the knockout animals. In contrast, high dose infection promoted uncontrolled bacterial growth in the lungs (160). Excessive production of chemokines can also be detrimental for the host as demonstrated by Rutledge et al. Transgenic mice overexpressing CCL2 are more susceptible to M. tuberculosis infection (156, 161). A critical component involved in the regulation of CC chemokine function is the D6 chemokine receptor or decoy receptor. This receptor is not specific for only one chemokine. It can recognize 15 different CC chemokines. Interaction between D6 and CC chemokines results in the formation of a receptor-ligand complex, which is internalized by the cells and causes degradation of the CC chemokines, recycling the decoy receptor to the cell membrane (162). In this way, the decoy receptor controls CC chemokine-driven inflammatory responses. M. tuberculosis infection in decoy receptor knockout mice causes excessive inflammation and reduces survival time (162). 30 Mucosal immunity in the respiratory tract More than 50 years ago Bull and McKee reported that specific immunity and protection against pneumococcal organisms could be generated in the nasal route after local instillation of organisms (163). The mucosal immune system has been an area of recent interest assuming that it is the most potential contributor of the host immunity since many infectious agents including M. tuberculosis come in contact with the host through the mucosal surfaces. Lungs, gastrointestinal tract and urogenital tract are covered by mucous membrane. M. tuberculosis gets access to human hosts via nasal mucosa and comes across the respiratory mucosa in order to be settled in the lungs. Therefore, lungs appear to be an important place where induction of immunological responses against TB is required. The upper respiratory tract comprising mucosal tissues and the lung parenchyma represents thin-walled alveoli. In the upper respiratory tract, epithelial lining containing ciliated cells and locally produced IgA antibodies prevent the entry of foreign invaders. IgA is the predominant immunogloubulin in most external secretions that exceeds that of all other immunoglobulin classes combined (164, 165). In humans, IgA comprises two subclasses: IgA1, dominant in serum and IgA2, mainly found in secretions. B cells produce IgA1 in response to protein antigens and IgA2 in response to polysaccharide antigens. In external secretions, both IgA1 and IgA2 are found in a different form called secretory IgA (sIgA), which is a polymeric form and is stable in the external secretions. The second compartment of the respiratory tract is largely associated with lymphoid tissues, containing both T and B cells. The lymphoid tissues within the bronchial walls form discrete structures, which resemble the Peyer's patches in the gut. This site is called bronchial-associated lymphoid tissue (BALT). The presence of BALT in adult human is controversial (166, 167), however, children do have BALT structure. Surveillance of the respiratory surfaces for antigens is primarily mediated by the resident airway mucosal DCs which capture antigens, upregulate CCR7 and migrate to the draining lymph nodes in order to present antigens to naive T cells. Antigen-specific T cells proliferate and differentiate into effector T cells that could home to the mucosal effector sites and contribute to the pathogen clearance. Antigen-specific CTL responses can be induced at the mucosal surfaces as reported by Gallichan et al (168). The CTL are long-lived, generated locally after mucosal but not systemic immunization and could migrate to the systemic compartments. Besides, the mucosal CTL responses, IFN-γ producing CD4 T cells have been found to be important for the mucosal protection against a variety of mucosal pathogens (169). Understanding of the mucosal immunity against M. tuberculosis has become an important event for the next 31 generation of TB vaccines. As a result, many studies have focused on mucosal vaccination using mycobacterial antigens in order to stimulate predominantly lung immunity, which is believed to be more effective against infection than that induced after systemic vaccination (169). Circumstantial evidence indicates that mucosal sensitization improves the level of protection and this could be due to the heightened cell-mediated immune responses found in the lungs (170-172). In addition, many studies reported on the passive transfer of IgA via intranasal route and improvement of protection against TB (122, 123, 173), which imply the potential role of IgA-mediated immunotherapy against TB. Fig. 4: Uptake of antigens and presentation to immune cells in the lung compartments. (Nature Rev. 2008;8:142-152, reprinted with permission from NPG ). 32 Vaccination against TB Frequently, vaccinations in humans are performed during the first years of life starting soon after birth. In fact, vaccines given to the neonates pose a huge challenge against the success due to the suboptimal maturation of the neonatal immune system. Vaccination against TB was introduced about a century ago. Unfortunately, failure of the BCG vaccination has been recorded and attempts are being taken to develop a new successful vaccine in order to ensure life-long protection against TB. The BCG vaccine ‘Mycobacterium bovis Bacille Calmette-Guérin’ is the complete name of the BCG vaccine, which was first introduced in 1921. Edmond Nocard first isolated a virulent strain of M. bovis called ‘lait Nocard’ which was transferred to the Institut Pasteur at Lille in 1901. Albert Calmette and Camille Guerin at the Institut Pasteur started culturing ‘lait Nocard’ organism with potato slices cooked in beef bile supplemented with glycerol for a duration of 3 weeks. This process was continued to a total of 230 passages by changing the culture medium every 2 weeks until 1921. The organisms lost their virulence over the culture period as tested in different animal models. The first BCG vaccination with the attenuated form of bacteria was performed in a newborn whose grandmother had pulmonary TB in 1921. This vaccinated individual remained free of TB throughout his life (174). From 1924 to 1926, BCG Pasteur strain was distributed to 34 countries and in 1927 more than 26 other countries were reported to receive the Pasteur strain. Since then many substrains of BCG were generated at different laboratories at various conditions and therefore today it has raised the question on the identification of the actual BCG strain in use (175). It is clear that BCG substrains are different from each other and also have different characteristics from the original strain. Comparative genomic analyses have first identified a gene coding for a protein MPB64, not found in some BCG substrains (176, 177). Subtractive genomic analyses were performed later to identify more specifically the deletion regions (RDs) among the substrains delivered before 1925 and after 1926 separating the BCG vaccine into ‘early’ and ‘late’ substrains. Strains that were distributed before 1925, had a RD1 deletion containing esat6 and cfp10 genes and strains delivered after 1926 had RD2 deletion which codes for mpb64 and cfp21 (Fig. 5). 33 Fig. 5: Evolutionary framework of BCG strains. (Vaccine 1999;17:915-922 , reprinted with permission from Elsevier Science Ltd.). The use of gene probe based experimental techniques helped to discover two copies of insertion sequence IS6110 in all the early substrains except Gothenburg substrain however, only one copy of IS6110 was found in the ‘late’ substrains. More recent investigation discovered that the deletion of insertion sequence is even not within the RD regions (175). Biochemical studies uncovered the differences among BCG substrains with regard to their lipid structures. Some of the substrains e.g. Gothenburg, Moreau and Tokyo strains have complex structure of mycolic acids containing methoxy groups attached to the mycolic acid. However, BCG Danish, Glaxo and Pasteur strains do not have such structure. Considering the genetic diversity and biochemical properties that the BCG substrains acquired during the attenuation process, it is probably not surprising that the protective efficacy of the BCG substrains declined after many passages. Studies reported that ‘early’ substrains including BCG Moreau and Tokyo are moderately immunogenic in animal studies than the ‘late’ substrains, which include BCG Danish and Pasteur (175). According to the genealogical tree of the BCG substrains, the ‘late’ substrains lost more regions than the ‘early’ substrains, however, this does not contribute to the immunogenicity or protective efficacy of the BCG vaccines (178, 179). As a result, World Health Organization recommended the most commonly used BCG substrains for future vaccination against TB (180). 34 Neonatal immunity and maternal-fetal interactions Immunization during the early life is required to protect infants from infectious diseases. However, during the early life, neonatal immune system is not sufficiently ready to respond to vaccine antigens and this leaves the neonates susceptible to infections. Since the neonates are often biased to develop Th2 type immunity and in some cases show unresponsiveness or immune tolerance, it may be of importance to consider special delivery protocols of vaccines/adjuvants to divert the immune responses. Induction of Th1 responses in the neonates has been made by using CpG-containing oligonucleotides (181), Freund’s complete adjuvant (182) or Titermax (183). The preferential polarisation of Th2 responses and failure to induce sufficiently high Th1 responses might be explained by the limited microbial exposure, lack of costimulatory activity by immune cells and suboptimal APC-T cells interaction in the neonatal life. In vitro studies have shown that neonatal T cells produce low levels of IL-2 and proliferate poorly in response to anti-CD3 stimulation, however, in the presence of anti-CD28 antibody and exogenous IL-6, neonatal T cells produce large amounts of IL-2 (184), implying a greater requirement of accessory cell signals for neonatal T cells. The reduced expression of MHC class II molecules in monocytes has been reported in utero and this could contribute to the impaired APC function (185). Similarly, human neonatal DCs have impaired synthesis of type I IFNs upon exposure to microbial components (186). Decreased expression of IL-12 by neonatal APCs and CD40-ligand by neonatal T cells has also been reported (187). A low level of CD40/CD40-ligand signals and insufficient amounts of IL-12 could limit the priming of the Th1 cells. Given the immune interactions between mother and fetus, investigators discovered several mechanisms, which may explain why the mother does not reject foreign fetus. One of the critical events is the failure of the trophoblast cells to express HLA class I and II molecules (188) and that may aid the fetal semiallogeneic graft in evading maternal immunologic responses. During the early pregnancy, nonclassical HLA molecules such as HLA-G and HLA-E have been found to be expressed by the cytotrophoblast cells. HLA-G has been shown to be important for the survival of fetus in the mother. The binding of HLA-G to the inhibitory receptors of maternal-uterine NK cells prevents NK cell-mediated cytolytic activity thereby, fetus remains unaffected (188). Moreover, a Th2 but not Th1-cytokine environment is critical for a successful pregnancy. It is now known that Th2 cytokine environment contribute to implantation of the embryo, development of the placenta, and survival of the fetus to term (189). 35 In addition, immunological contribution from the mother to the fetus or to the neonates could also block immune activation process in the neonates. For example, studies showed that maternal antibodies could suppress vaccine responses against tetanus, diptheria toxoids (190, 191), haemophilus influenzae type b (Hib) conjugates (192, 193) and hepatitis A antigens (194). This could be overcome by repeated vaccination with diptheria-tetanuspertusis-polio or Hib vaccines. The transfer of passive immunity from the mother to the baby via placenta could be present in the neonates until the first couple of months. These antibodies disappeared by six months of age (195) and therefore have less influence on the repeated vaccination. Of note, the presence of maternal antibodies has no influence on neonatal T-cell proliferation and cytokine production as observed in mouse models with tetanus toxoid or measles vaccines (196, 197). It is possible that the neonatal immune cells are not ready to respond sufficiently to vaccine antigens since both qualitative and quantitative differences are noticed among innate and adaptive immune cells from newborns and adults (186, 198). However, mounting evidence indicates that the condition of the vaccines including delivery or presence of strong adjuvant could shape the neonatal immune system (187). BCG vaccination during the neonatal age is one of the classic examples that could induce sufficiently adult-like immune responses (199). Moreover, immune activation or priming of the immune system could also occur as early as during the gestational period when fetal immune cells could be activated by antigens transferred from the mother to the fetus through placenta (200, Paper III). This prenatal exposure to antigens could impact on higher immune responses during the postnatal life, which has been reported (201, Paper III). Prenatal exposure to mycobacterial antigens enhanced postnatal immune responses, which were similar to that observed in adults (201). 36 Failure of BCG and progress in new TB vaccine development Unfortunately, the BCG vaccination has been reviewed unsuccessful against adult pulmonary TB but effective against childhood tuberculous meningitis and miliary disease. Therefore, BCG vaccination is still recommended in many countries with high prevalence of TB. There is no single cause that could explain why the BCG vaccination failed to deliver enough protection. A combination of many factors perhaps contributes to the lower efficacy of the BCG vaccination. For example, genetic differences within and among host populations, varying levels of malnutrition among host populations, virulence differences among M. tuberculosis strains, endogenous reactivation of persistent infection versus exogenous reinfection, effects of environmental mycobacteria on the host immune response to BCG, and methodological differences among the clinical trials. In addition, the BCG-derived immunity wanes with time, which might not be due to the decreased levels of Th1 immunity or to the factors mentioned above. It has been suggested that other mechanisms like the induction of inappropriate Th2 immunity by helminth infection (202) or regulatory T-cell activity (203) are likely to contribute to the poor Th1 immunity. Since the protection offered by the BCG vaccination against adult pulmonary TB is variable, many approaches have been pursued to achieve a new successful vaccine, which includes auxotrophic vaccines, rBCG, DNA vaccines and subunit vaccines. The most commonly used experimental animals are mouse and guinea pig that have been vaccinated with the newly developed vaccines in order to obtain some basic information such as the ability to control bacterial burden, lung pathology and to improve survival period. A summary of the TB vaccines tested to date is depicted in Table 1. 37 Table 1: List of TB vaccines tested in animal models for evaluation of protective efficacy Subunit vaccines Vaccine type Single antigens 1. HBHA 2. Ag85B 3. ESAT-6 4. TB10.4 Fusion antigens 5. Ag85B-ESAT-6 6. Ag85B-TB10.4 7. Mtb72F (MTB32, 39) 8. STCF 9. CFP-10 10. DNA-85A 12. DNA-85B 13. DNA-ESAT-6 14. rAd vector--Ag85A, Ag85B, and TB10.4 Antigen complex DNA vaccines Auxotrophic Combination vaccine (prime-boost) Live vaccine Name of the vaccine Recombinant BCG (rBCG) DNA primefusion protein boost Viral vector prime- fusion protein boost BCG primeprotein boost 15. Auxotrophs BCG 16. Over-expressing Ag85 17. Coexpressing Ag85BESAT-6 18. ∆ureCHly+rBCG 19. DNA-Ag85A prime …Ag85B-ESAT-6 boost Protection compared with BCG (Mouse) =BCG <BCG <BCG <BCG Survival compared with BCG (Guinea pig) ≥BCG* ≥BCG* <BCG =BCG* <BCG <BCG <BCG 204 205 206 205 207, 208 209 210 211 212 213, 214 <BCG 215 <BCG >BCG >BCG 216 217, 218 219 =BCG 220 221 =BCG 221 >BCG 20. MVA-Ag85A prime…Ag85B-ESAT-6 boost 21. BCG… Ag85A boost 22. BCG… Ag85B-ESAT-6 boost 23. BCG… Ag85B-TB10.4 boost References >BCG >BCG (i.n.) 222 170 >BCG 223 BCG prime24. BCG… MVA85A boost 221 >BCG viral vector 25. BCG…Ad85A 172 >BCG (i.n.) BCG prime26. BCG…ESAT-6 224 >BCG DNA boost 225 27. BCG/Ag85B-ESAT-6--BCG/protein >BCG Ag85B-ESAT-6 prime-protein boost *Not significantly better; i.n. intranasal, Ag, antigen; STCF, short-term culture filtrate; CFP-10, culture filtrate protein, 10 kDa; rBCGDUre:CHly+, rBCG expressing listeriolysin Subunit vaccines Besides showing high efficacy, a vaccine has to be safe for human use and it is generally considered that subunit vaccines are safe than live or attenuated vaccines. Though subunit vaccines are considered to be safe, one of the shortcomings of subunit vaccination is that the candidate antigen alone could not stimulate enough the host immune system and therefore 38 induces suboptimal immune responses. Thus, most subunit vaccines require adjuvant. Unfortunately, progress of subunit vaccine development is impeded due to the lack of safe and effective adjuvants. However, searching for a safe and effective adjuvant for human use is continuing and very recently squalene-containing adjuvants have been found to be safe in human when used in two H1N1 vaccines developed by Novartis and Glaxo-Smith Kline (226). Also, IC31(R) adjuvant has been found to be safe in humans when administered with Ag85-ESAT-6 fusion antigen (227). The development of subunit vaccines against TB is being focused much in the recent times since the genomic sequences of M. tuberculosis are available now (228) and it is believed that a key antigen of the M. tuberculosis might induce protective immune responses. Several single mycobacterial protein antigens including Ag85 complex (30-32 kDa), ESAT-6, CFP-10 and TB 10.4 (belong to the esat-6 gene family), 19 and 38 kDa lipoproteins, HBHA have been tested in preclinical studies using mouse models and have been found to be promising although none of them induced better protection than the BCG vaccination. Vaccination with HBHA has been shown to be as effective in controlling bacterial burden as the BCG vaccination (204). Therefore, HBHA has been demonstrated as a potent vaccine candidate given also the fact that HBHA specific immunity was found in the healthy infected individuals but not in the individuals with active disease (229). In order to prioritize the subunit vaccination, instead of adding one single antigen in the vaccine formulation, polyprotein antigens have been used, which showed great promise for future vaccination. This has been used in vaccines against other infectious diseases such as malaria (230-232), leishmaniasis and hepatitis. Fusion or polyprotein antigens in the vaccines could induce better protection than any of the components of the fusion antigen tested individually. Mycobacterial Mtb72F, a 72-kDa polyprotein genetically linked in tandem in the linear order Mtb32(C)-Mtb39-Mtb32(N), Ag85B-TB10.4 and Ag85B-ESAT-6 are most used fusion antigens that conferred protection comparable to that induced by the BCG vaccination (208, 209, 233). All three candidates have entered clinical trails. DNA vaccines DNA vaccination is one of the new strategies that has drawn focus on new TB vaccine development. Instead of immunizing with the foreign antigen, a gene of interest is inserted into a vector and finally the vector is taken up by the host cells. Using host cell machineries the gene of interest is expressed and the host develops immunity against this specific antigen. DNA vaccine against mycobacteria was first introduced in 1994. The J774 macrophage cell line was transfected with the M. leprae Hsp65 gene and administered into syngeneic 39 (BALB/c) mice (234). This way of immunization induced protection against M. tuberculosis infection (235). In mice, DNA vaccines targeting mycolyl transferase enzyme, Ag85A or 38kDa lipoprotein have been shown to induce some level of protection against M. tuberculosis infection (213, 214, 236). DNA vaccination could generate both humoral and cellular immune responses as described by Huygen et al (236) and the immune responses were found to be mixed Th1/Th2 types (237). Although many attempts were taken in the preclinical settings using mouse models and the outcomes were promising, experiments in the larger animals like guinea-pigs or non-human primates showed disappointing results with DNA vaccination (238). Auxotrophic vaccines Immunocompromised individuals are at greatest risk of developing disseminated TB after receiving the conventional live BCG vaccine. Therefore, auxotrophic mutants of BCG or M. tuberculosis have been introduced in order to reduce the virulence without affecting the immunogenicity in vaccinated animals. Auxotrophic mutants of BCG, require specific amino acid to grow, reported to be safe in experimental animals (216). Auxotrophic vaccines from M. tuberculosis have also been made during the past years, however, there are concerns about reversion of virulence and thus their use requires more investigation. Although auxotrophic vaccines are believed to be safe at least in the experimental animal models, a major problem seems to be the short period of immunological memory (239) due to the limited duration of replication in the host (216, 240). rBCG vaccines Recombinant DNA technology has created an opportunity to reinsert the missing genes in the BCG vaccine as well as in inducing the expression of one target gene. Several strategies have been followed to develop vaccines against TB. The rBCG expressing RD1 genes comprising ESAT-6 and CFP-10 has been shown to be more protective than the wild-type BCG (241). Bao L et al developed two rBCG vaccines containing ESAT-6 fused with HSP-60 and ESAT6 with a secretory sequence. Both of them showed enhanced protection against M. tuberculosis infection in animal models (242) however, none of them showed significantly better level of protection compared with the wild-type BCG vaccination. rBCG designed for overexpression of one single gene has been found to be attractive. For example, rBCG30 constructed with mycobacterial Ag85B, which is a 30kDa enzyme involved in outer cell wall synthesis, appears to enhance survival time significantly better than 40 the conventional BCG vaccine upon challenge with a highly virulent strain of M. tuberculosis (218). Phase I clinical trial with rBCG30 vaccine has been completed in 35 healthy adult volunteers and found to be safe and immunogenic (243). In preclinical studies, rBCG expressing listeriolysin (rBCGDUre:CHly+) was reported to induce better protection than the conventional BCG vaccination (220). Listeriolysin O is expressed after vaccination with rBCGDUre:CHly+. Listeriolysin induces perforation in the membrane of early phagosome and thereby promotes leakage of some BCG into the cytoplasm. The basic principle of this type of vaccine is to enhance both CD4 and CD8 type T cell responses by augmenting MHC I and MHC II dependent antigen presentation. It has been postulated that listeriolysis not only promotes pore formation but also apoptosis of the infected macrophages. Releasing antigens form apoptotic blebs that are taken up by DCs and increase antigen presentation to T cells followed by a mechanism called ‘cross-priming’. Listeriolysin functions in an acidic environment. However, BCG secreting urease could block the acidification process. Therefore, the urease gene was deleted in the rBCGDUre:CHly+ vaccine in order to facilitate the acidification. When tested in immunocompromised SCID mice rBCGDUre:CHly+ was found to be safe (220). Combination vaccines (prime-boost) Prime-boost vaccination strategy is probably the best approach in terms of achieving high level of protection as observed in many pre-clinical studies. Different forms of prime-boost vaccination have been tested so far i.e. protein-protein, DNA-protein, BCG-protein antigens. As many people around the world already received the BCG vaccine, and their immune system is already primed, the future vaccination strategy should consider boosting of the BCG-derived immunity. In the combination of BCG priming and boosting with protein antigens, protein antigens could be delivered together with adjuvants or in the form of naked DNA or viral vector. Skeiky and Sadoff (244) have explained how the immune responses are generated following priming with BCG or rBCG and boosting with recombinant protein in adjuvant or viral vector (Fig. 6). This heterologous prime-boost vaccination could preferentially induce antigen-specific memory T-cell expansion against some epitopes shared by both the priming and the boosting antigens. 41 Fig. 6: How does prime-boost vaccination work? Modified from Nat Rev Microbiol. (2006;4:469-476, reprinted with permission from NPG) Whichever the combination of prime-boost vaccination was followed, priming with BCG and boosting with another antigen (single or tandem) displayed significantly higher protection than the BCG vaccination alone. McShane et al progressed with recombinant modified vaccinia virus Ankara (MVA) expressing Ag85A (MVA85A) as a BCG-boost vaccine in clinical trials. This was the first candidate subunit vaccine in clinical trials to boost BCG immunity and found to be efficient in enhancing and prolonging antimycobacterial immunity (221, 245-247). A subunit BCG-boost vaccine comprising Ag85B and TB10.4 (HyVac4) delivered as a fusion molecule and formulated with the adjuvant IC31 has been shown to provide higher protection than BCG vaccination alone in the more stringent guinea pig model of pulmonary TB (223). This vaccine was reported to be safe and will be progressed for clinical studies. Single mycobacterial antigen HBHA has been evaluated in mouse models as BCG-boost vaccine and found to increase immune responses and protection (Paper II, 248). Immune correlates of protection (Multifunctional T cells) There are a number of different types of T cells that has been named as naive T cells, memory T cells and within the memory-cell population it is further classified as effector memory Tcells or central memory T cells. All these groups of cells are differentially regulated and 42 thereby produce different cytokines, suggesting their heterogeneous effector functions and also their ability to homing different tissues (249, 250). Multifunctional T cells are primarily characterized by their ability to produce more than one cytokine concomitantly. Multifunctional T cells are not only restricted to the CD4 T cell population, CD8 T cells can also be multifunctional. CD8 T cells with multifunctional characteristics correlated better with the control of HIV-infection (251) because IL-2, TNF and IFN-γ triple positive T cells produced more IFN-γ than double or single cytokine producing cells implying better effector functions of polyfunctional T cells. Multifunctional T cells have been suggested to be important in protection although very little is known about their mechanism of action. This has been investigated with much attention especially in vaccine research. The hypothesis is that detection of a single cytokine producing cell population, which might have limited functional activity is probably insufficient in the process of characterizing immune correlates of protection. Several studies have already addressed the importance of multifunctional T cells in hepatitis B virus vaccine (252) and HIV vaccine (253) development, vaccinia-induced responses (254) and also in Leishmania major infection (255). Assessment of the frequency of Th1 cytokine producing cells, mainly CD4 T cells expressing IFN-γ, has been considered the principle correlate of immune protection against TB but many studies have recently showed that this was insufficient (4, 256). It has been demonstrated in animal models of TB vaccine studies that the presence of multifunctional T cells coexpressing multiple cytokines correlated better with the vaccineinduced protection when animals were challenged with M. tuberculosis (257). Contrasting results have been obtained by Tchilian et al; systemic multifunctional T cells do not correlate with the protection in a murine model of prime-boost immunization study (258). Forbes et al compared mucosal and systemic routes of vaccination in a murine model of TB and found that multifunctional Th1 cells in the lungs but not in the spleen correlate with the protection (259). Thus it remains to be determined the exact phenotype of the multifunctional T cells and their subtypes which could be of importance in the characterization of immune correlates of protection. Route of vaccination (mucosal versus systemic) Mucosal vaccination promotes induction of local and systemic immune responses but vaccination via systemic routes (subcutaneous or intramuscular) mostly generates systemic immune responses, which are not sufficiently effective against pathogen invasion at the mucosal sites. A vast majority of pathogens invade via mucosal surfaces and therefore it is 43 considered that new-generation vaccines should target mucosal route for the induction of local as well as systemic immunity. Examples include enteric infections caused by Helicobacter pylori, Vibrio cholerae, enterotoxigenic Escherichia coli (E. coli), Shigella spp., respiratory infections caused by M. tuberculosis, Mycoplasma pneumoniae, influenza virus and respiratory syncytial virus; and sexually transmitted genital infections caused by HIV, Chlamydia trachomatis, Neisseria gonorrhoeae and Herpes simplex virus (169). It is becoming clear that the development of a broader range of mucosal vaccines requires the development of effective and safe mucosal adjuvants. The best-studied and potential mucosal adjuvants in experimental animal models are cholera toxin (CT) and E. coli heat-labile enterotoxin (260, 261). Non-toxic forms of CT such as cholera toxin B subunit (CTB), active cholera toxin A1 subunit linked to specific APC-binding protein derived from Staphylococcus aureus protein A (CTA1-DD) have been developed and proven to be a very efficient and safe adjuvant (169). Bacterial DNA or synthetic oligodeoxynucleotides containing unmethylated 'CpG motifs' (CpG ODN) has been found to be promising as a mucosal adjuvant when CpG ODN was linked to the B subunit protein of CT (262). The mucosal route of vaccination against TB including i.n. or oral delivery of antigens or attenuated M. tuberculosis has been tested in many preclinical studies. Oral mucosal vaccination has several disadvantages such as it requires much higher doses of antigens, increases the exposure to low pH and a broad range of enzymes. In contrast, i.n. mucosal vaccination could significantly reduce these limitations and sensitize better the immunological compartments at the nasopharyngeal tissue and other innate organs all the way down to the respiratory tract. Studies in mouse models have shown that the mucosal (i.n.) route of vaccination is more effective than the systemic route of vaccination even when BCG is used (263). Instead of delivering live organisms via nasal route, delivery of purified single or fusion antigens, and DNA vaccines have received much research focus as a potentially useful concept. Rodríguez A et al have shown that i.n. immunization induces both mucosal and systemic immune responses while intraperitoneal immunization induces only systemic immune responses (264). Santosuosso et al demonstrated that i.n. but not intramuscular vaccination with adenoviral-based vaccine (AdAg85A) showed protection against pulmonary M. tuberculosis challenge (265). In a prime-boost immunization protocol, protection by BCG prime immunization was effectively boosted by AdAg85A administered i.n. and that correlated with the increased levels of IFN-γ producing CD4 and CD8 T-cell responses in the airway lumen (172). Subcutaneous or intramuscular boosting with BCG or AdAg85A respectively failed to impart such protection. Similarly, fusion antigens Ag85B-ESAT-6 44 delivered via i.n. induced strong T cell responses in the spleen, blood, draining lymph nodes from the nasal cavities whereas subcutaneous immunization with the same antigen failed to induce similar immune responses in the lymph nodes (170). Likewise, mucosal administration of Ag85B-ESAT-6 increased protection significantly against M. tuberculosis infection in the BCG-primed mice compared with the BCG-vaccinated nonboosted mice (170). 45 Diagnosis of TB Yet, there is no reliable technique available for the diagnosis of TB. Thus, a secure and quick test is of utmost importance in order to interrupt the transmission of TB. Methods that are being used today in the diagnostic laboratories or under development show limitations in producing accurate results. Although a number of diagnostic tests are in use none of them are optimal - either slow to perform, less sensitive or difficult to establish in resource-limited countries. Diagnosis of TB infection has been truly dependent on microbiological tests since the M. tuberculosis was identified. Currently, immunological and PCR-based tests are available which show an improvement in the detection process. Since a successful penetration of M. tuberculosis in human host could lead to either latent infection or active disease, development of diagnostic techniques have also focused separately on these two different outcomes. Classical methods Microscopy and culture Microscopic visualization of M. tuberculosis is extremely fast, inexpensive and results can be delivered within hours. This method has been extensively used during the past decades to diagnose mainly active TB disease. A patient with active TB usually suffers from coughing and produces sputum and it was observed that mycobacteria are present in the sputum. Ziehl and Neelsen introduced a special bacteriological stain, called acid-fast stain, for microscopic visualization of the bacilli. Unfortunately, several shortcomings hinder its use for accurate diagnosis of TB. It requires 5x103 bacilli/ml concentrations in the sputum samples to be able to detect them (266), which is nearly impossible to get from the children because they do not produce enough sputum. Acid-fast staining test cannot be used to monitor drug susceptibility or resistance which therefore delays the treatment procedure. Some modifications prior to staining including cytocentrifugation or overnight sedimentation have been found to increase the concentration of bacilli in the smear and results in higher sensitivity (267). Conventional culture methods to isolate M. tuberculosis from the clinical samples are most definitive diagnostic procedures for TB. Mycobacteria can grow in egg based solid medium such as Lowenstein-Jensen medium or agar based, Middlebrook 7H10 or 7H11 medium and also in liquid medium such as Middlebrook 7H9 broth. Various types of samples such as sputum, bronchial washings or non pulmonary samples can be used to cultivate mycobacteria in the laboratory. The major difficulty of laboratory cultivation of mycobacteria 46 is slow growth-rate, which essentially takes an average of 4 weeks and examination of drugresistance or susceptibility tests requires additional 4 weeks. Tuberculin skin test Tuberculin skin test is performed by intradermal injection of purified protein derivative (PPD) tuberculin. This test is widely used to diagnose latent infection and the response against PPD is dependent on T-cell function. PPD tuberculin is a glycerol extract obtained from the culture of tubercle bacillus. In 1890, Robert Koch first described the PPD tuberculin. After injecting the PPD into the forearm, an induration is measured in millimetres within 48-72h. Although this test is simple and easy to perform and has been used in many countries around the world unfortunately the test results can be positive for the people infected with mycobacteria other than the M. tuberculosis. More importantly, BCG-vaccinated people react against PPD and become positive in the test. On the other hand, people with anergy (unable to respond to foreign molecules) most commonly with HIV infection failed to show enough immune reaction and could be diagnosed negative in the test (268). Radiology or chest X-ray Chest X-ray allows visualization of infiltrates or consolidations and/or cavities in the upper lungs. Abnormalities on the radiograph do not indicate TB disease however; it can be suggestive of TB if a person is positive for PPD skin test. Chest X-ray can also be useful in monitoring the treatment progress of the disease. Radiology or chest X-ray is used in conjunction with PPD skin test and microscopy for acid-fast bacilli. New methods BACTEC radiometric system The BACTEC (Becton-Dickinson) radiometric system introduced relatively faster detection of mycobacterial growth compared to the conventional culture assays. In this assay system, radioisotope, 14 C labelled palmitic acid containing 7H12 medium is used to culture the mycobacteria. Palmitic acid substrate is used up by the bacteria and produce 14CO2, which is detected in an ionic chamber with electronic detector in the BACTEC instrument. Instead of waiting for bacterial colony formation, released 14 CO2 from the culture is used to calculate growth index. BACTEC radiometric system can be used efficiently to test drug-susceptibility. Risk of having exposure of radioisotopes and also the equipment costs preclude its implementation as a routine diagnostic protocol in the developing world. 47 Microscopic-observation drug-susceptibility (MODS) assay In order to obtain result as early as possible with high sensitivity, a modified method with the combination of microscopic and culture techniques has been evaluated recently (269). More importantly, this combined method is very useful to monitor drug-susceptibility when the culture is positive. MODS assay has been developed based on the bacterial growth in liquid medium since bacteria in the liquid medium grow faster than the solid agar. Also, it is easy to visualize microscopically early the characteristic cord formation of M. tuberculosis in the liquid medium. Moore et al found that the sensitivity of MODS assay is higher (97.8%) than the automated mycobacterial culture (89%) or Lowenstein-Jensen culture (84%) techniques (269). Accomplishment of the MODS test including drug-susceptibility test is faster (2 weeks) than automated mycobacterial culture (6-7 weeks) or Lowenstein-Jensen culture (13-14 weeks) techniques. Although MODS assay is a fairly cheap and simple method for the diagnosis of TB, it requires a very skilled laboratory technician to identify the organism microscopically. Since the culture needs to be handled often, it poses biosafety risk for the laboratory staffs. Nucleic acid amplification assay Nucleic acid amplification assay by polymerase chain reaction (PCR) is a modern approach for rapid diagnosis of TB. This method allows exponential increase of the copy of target DNA from the specimens through a process of multiple cycles of denaturation, annealing and polymerization. The PCR assay is simple and rapid; results can be obtained within a day of DNA isolation from the clinical samples. Among several target genes identified for the detection of M. tuberculosis, IS6110 is the most common one, which is present up to 20 times in the genome. Some other targets including 65 kDa heat-shock protein gene, the 126 kDa fusion protein gene, 16S-23S spacer region and the gene encoding the β-subunit of RNA polymerase have also been tested for the identification of M. tuberculosis (270). Although the PCR-based methods are highly sensitive and specific, major challenges are remained in the implementation of this technique that include skilled workers, PCR machines and biosafety level-3 facilities, which are not cost effective for many developing countries. Immune-based tests Serological: Detection of specific immune complexes, antibodies in serum or circulating TBantigens have been a new attempt for the rapid diagnosis of TB. The most common format of serological tests is enzyme-linked immunosorbent assay (ELISA) or immunochromatographic 48 tests. An accurate serological test would be advantageous over the conventional microscopy or culture-based assays. Serological tests are quick to perform and especially needed for the patients who rarely produce sputum (children) and also are smear negative. The most common format of serological tests relies on the detection of antigen-specific antibody responses in sera of TB patients. A meta-analysis of 254 studies has recently been performed to evaluate the performance of single antigens and multiple-antigen combinations for the serodiagnosis of pulmonary TB (271). A total of 13 distinct antigens (recombinant 38kDa, native 38kDa, MPT51, malate synthase, CFP-10, TbF6 polyprotein, Ag85B, α-crystallin, 2,3Diacyltrehalose, 2,3,6-triacyltrehalose, sulfolipid I, cord factor, TbF6 plus MPT32) and several multiple-antigen combinations were included. Compared with the single antigenbased serological tests (median sensitivity 53%) multiple-antigens used for the detection of antibodies showed higher sensitivities (median sensitivity 76%). Examination of the immunoglobulin classes revealed that detection of IgG and/or IgA antibodies provided higher sensitivities than that used for IgM detection (271, 272). Unfortunately, the detection of antigen-specific antibodies in sera was not sufficiently sensitive/specific when compared with the gold standard culture-based methods. Furthermore, individuals exposed to environmental mycobacteria or upon BCG vaccination develop antibodies against common antigenic epitopes. This generates difficulties in the interpretation of the results. Hence, antigens such as ESAT-6 and CFP-10, which are present in M. tuberculosis but not in BCG have been used to detect antibodies. Unfortunately, the use of ESAT-6 and CFP-10 is also questioned due to the fact that these two proteins are not exclusive for M. tuberculosis (273) since orthologues of ESAT-6 and CFP-10 are present in M. leprae and M. smegmatis. Recently, Rv3425, a member of the PPE family of proteins, encoded by RD11 of M. tuberculosis has been demonstrated as a potential antigen for serological diagnosis because Rv3425 is missing from BCG and all virulent M. bovis strains tested (274). Rv3425 specific IgG responses were significantly higher in patients with active disease compared to the BCG-vaccinated healthy controls (274). The detection of circulating TB antigens using monoclonal antibodies has been a promising strategy for the diagnosis of active TB. Among many others, El-Masry et al developed a modified ELISA, which is called Fast-Dot ELISA (FD-ELISA), for the detection of circulating 20kDa TB antigen (275). FD-ELISA has been reported to be simple, rapid and highly sensitive (90.8%) for the detection of TB antigen in serum samples of TB patients. However, further studies are required to improve reproducibility of the FD-ELISA-based diagnosis in a large population. 49 T cell-based cellular immune response: The oldest and widely used method based on the cellmediated immune response is the tuberculin skin test. A major drawback of this test is that it produces false positive results in people exposed to the environmental mycobacteria or vaccinated with BCG. Thus, efforts have been made to develop new tests based on the release of IFN-γ by T cells upon in vitro stimulation with antigens specific to M. tuberculosis. Antigens, ESAT-6 and CFP-10 have been used for such stimulation and a great success was observed in the detection of latent infection with more accuracy than the PPD skin test (276). This diagnosis method is based on the assumption that T cells from sensitized individuals produce IFN-γ when they re-encounter antigens of M. tuberculosis. The most promising and readily available test based on the IFN-γ release assay is QuantiFERON-TB Gold, which is the advanced version of QuantiFERON-TB. QuantiFERON-TB Gold test was certified in 2005 by United States Food and Drug Administration. A positive test indicates that the M. tuberculosis infection is likely and a negative test indicates that infection is unlikely. Analysis from several studies have found that QuantiFERON-TB Gold test has 97.7% specificity (277) in the diagnosis of latent TB infection but it showed variable sensitivity (55-88%) in the diagnosis of active TB infection (277). As stated previously, antigens ESAT-6 and CFP-10 are not truly specific for M. tuberculosis, therefore it is likely that the test results might be influenced in some cases if the subjects are exposed to other mycobacteria. On the other hand, a significant number of expertise, trained technicians and necessary equipments are required to run the test smoothly within a short window period 16-24h and interpret the results. The test is still under evaluation in the diagnosis of TB in children and patients with HIV infection. Non-invasive methods: Non-invasive, needle-free, immune-based methods for the diagnosis of TB are of utmost importance in the developing world to limit the spread of the infectious diseases. In this case, saliva samples from TB patients can be checked for the presence of specific sIgA. This might be possible since previous studies have shown in mouse models that antigen-specific IgA antibodies were increased after mycobacterial infection or i.n. immunization with mycobacterial antigens (264, 278). In a human study, Araujo et al succeeded in the detection of 38kDa-specific sIgA in saliva samples of TB patients. Unfortunately, this study reported very low sensitivity (36.1%) (279). Another non-invasive immune-based method has been described by Hamasur et al for the rapid diagnosis of TB based on the detection of mycobacterial LAM in urine samples of TB patients (280). A catchup ELISA and a dipstick test were used to detect LAM antigen at concentrations of 1 ng/ml 50 and 5 pg/ml respectively. The sensitivity was 81% and the specificity was 87%. Few laboratories in the developing countries are well equipped with the necessary safety cabinets to be able to work safely, thus, this diagnostic method based on the detection of antigen in urine samples would be extremely valuable. Biomarker (s) of infection There have been continuous efforts to rectify the available diagnostic methods for an accurate diagnosis of TB. Biomarkers are biological features or substances that can be used as indicators of infection. This definition of biomarker has widened the search for specific parameters. Recently, IFN-γ-inducible protein 10 (IP-10/CXCL10) has been evaluated as a potential marker for active TB. It has been reported that IP-10 is secreted by lymphocytes and monocytes after antigenic stimulation of whole blood cells and could be useful as a biomarker for active TB in children (281). IP-10 is not a specific marker of TB since IP-10 levels are also increased in patients with autoimmune disease (systemic lupus erythematosus, thyroid disease), acute coronary syndrome, cerebral malaria and allergy (282-285). Volatile metabolites from M. tuberculosis organisms can be used as biomarkers of infection. Compounds such as methyl phenylacetate, methyl p-anisate, methyl nicotinate and ophenylanisole have been detected in cultures before colonies were appeared. Phillips M et al demonstrated the significance of volatile compounds in the diagnosis of TB based on the fact that detection of volatile compounds could differentiate between ‘sick’ and ‘healthy’ subjects including infected and non-infected individuals (286). Cytokine levels in broncho-alveolar lavage (BAL) could be good markers of infection. TNF, IFN-γ and IL-2 levels in BAL of patients with smear-negative pulmonary TB are increased significantly compared to the other pulmonary disease (287). Moreover, other candidate biomarkers for TB infection such as lactoferrin, CD64, and the Rab33A (member of the Ras-associated GTPase) have recently been suggested (288). Expression of CD64 on monocytes has been found higher in TB patients than M. tuberculosis-infected healthy subjects. Similarly, a higher gene expression of lactoferrin and Rab33A was reported in TB patients. However, these molecules whether or not specific for TB are yet to be defined. Nevertheless, these molecules may play a role as part of a panel of diagnostic biomarkers. 51 PRESENT STUDY Aims TB remains a global problem even though it is a curable disease. The goal of eliminating TB is largely dependent on the development of simple and rapid diagnostics, drugs and vaccines. In order to improve the diagnosis and vaccination against TB, the overall aim of this study was to understand the mucosal immune responses in the respiratory tract upon mycobacterial infection or vaccination with mycobacterial antigens in experimental murine models. Our specific objectives were: To detect immune responses locally (lungs) and systemically (blood) in mice upon mycobacterial infection and to identify immunological parameters or biomarkers associated with the infection (Paper I) To investigate the priming effect of neonatal BCG-vaccination in a heterologous prime-boost immunization protocol (Paper II). To examine the effect of maternal gestational treatment with mycobacterial antigens on postnatal immunity in the offspring (Paper III). To determine the importance of innate immunity in mycobacterial antigen recognition, processing and presentation for the induction of immune responses and protection (Paper IV). 52 Methodology The methods used for paper I-IV are described in the respective ‘Materials and methods’ section of each paper. The following methods were used: -ELISA (Enzyme-linked immunosorbent assay) -Flowcytometry -Gel-electrophoresis -Fluorescence microscopy -Isolation of mononuclear cells from mouse lung and spleen -In vitro antigen restimulation -In vitro restimulation with BCG- or antigen-pulsed bone-marrow derived macrophages -Statistical analyses All in vivo experiments were conducted in mice. All experiments were done in accordance with the ethical guidelines available at Stockholm University. 53 Results and Discussion Paper I Increased levels of immunological markers in the respiratory tract but not in serum correlate with active pulmonary mycobacterial infection in mice The mucosal immune system is highly compartmentalized; origin of activation of immune cells determines the fate of effector and memory cells. Cells activated in the NALT and Peyer’s patches preferentially home to the nasal mucosa and to the intestinal mucosa respectively (289). This unique characteristic of mucosal immune responses led us to hypothesize that detection of immune biomarkers in the respiratory mucosal secretions but not in serum would correlate better with mycobacterial infection since nasal mucosa is the natural path for penetration of the host by M. tuberculosis. The major findings of this study are: a) Active mycobacterial infection, but not exposure to non-replicating mycobacteria resulted in elevation of IL-12, IFN-γ and sTNFR in BAL independently of the route of infection. Serum levels were not equally conclusive. b) Reactivation of controlled BCG infection resulted in increased bacteria growth in the lungs and in increased levels of sTNFR in BAL. c) Active infection, but not exposure to non-replicating mycobacteria resulted in production of antigen-specific IgG or IgA in BAL. One of the major problems of the immune-based diagnosis is that it fails to discriminate between active infection and the exposure to mycobacteria or BCG vaccination in individuals. In order to improve the situation, in this study we chose some immune markers namely sTNFRs (sTNFR1 and sTNFR2) and cytokines (TNF, IL-12 and IFN-γ) and assessed whether the levels of any of these markers correlated with active but not exposure to mycobacterial infection. To test this, we modelled the natural route of infection by infecting mice i.n. with live (replicating) or dead (heat-killed, non-replicating) BCG. To mimic the exposure of mycobacterial antigens, we also treated mice with BCG-lysate (soluble antigens). The important observation from this study was that i.n. infection with live BCG but not with nonreplicating BCG or BCG antigens induced elevated levels of sTNFRs, IL-12 and IFN-γ in the BAL (Fig. 1 in Paper I). A strong positive correlation was observed between the bacterial 54 growth in the lungs and the levels of sTNFR1 and sTNFR2 (r 0.7 and r 0.68, respectively) (Fig. 1d-e, in Paper I). Unlike sTNFRs, levels of IL-12 showed a poor association with bacterial growth (r 0.48). In contrast, although the levels of IFN-γ increased after infection and decreased when the bacterial growth was controlled, the peak of IFN-γ levels did not coincide with the peak of bacterial growth (Fig.1c, in Paper I). To evaluate whether the responses were restricted to the i.n. route of infection, we measured cytokines and cytokine receptor after infecting mice intravenously (i.v.). Similar results were obtained even after i.v. infection. In contrast to the data obtained from BAL, results from the serum analysis showed that infection with live BCG as well as treatment with non-replicating BCG caused increased levels of IL-12 and sTNFRs in serum, however, there was no correlation found between the serum cytokine or sTNFRs and the bacterial growth in the lungs (Fig. 2a-c, in Paper I). In general, upon exposure to M. tuberculosis, lung macrophages in the host phagocytose the bacilli and thereafter host immune responses are initiated to eliminate the bacilli. In response to infection, macrophages produce cytokines such as IL-12 and TNF and T cells are recruited to the site of infection and produce IFN-γ. A combination of TNF and IFN-γ mediated activation of macrophages leads to the formation of granuloma (290). The findings from Paper I strongly emphasise on the fact that the measurement of immune responses particularly cytokines or sTNFRs in the respiratory mucosal secretions but not in the blood might be better correlates of infection. The relevant supports came from the experiments when we tested non-replicating BCG or BCG-lysate i.n. for the induction of immune responses. Daugelat and colleagues have reported that infection with live- but not killed-BCG resulted in the activation of a type of T cells, which are able to recognize secreted antigens (291). The difference between the live- and dead-BCG lies on the fact that live BCG could replicate and secrete antigens and therefore stimulate a wide range of immune cells compared to the non-replicating BCG. In addition, the responses in the lungs after live-BCG infection were even detectable in the blood but did not correlate with the bacterial burden neither in the spleen nor in the liver. In recent times, several studies have focused on the detection of immune parameters locally in the lungs, which might be good correlates of infection. Expression of IL-12 and IFN-γ mRNA in the BAL (292) and elevated levels of cytokines such as IFN-γ, IL-12, IL-1β, IL-8 and TNF produced by broncho-alveolar cells were described to be associated with active pulmonary TB (293). In addition to the cytokine levels, cytokine receptors, such as sTNFR, have been reported to be a sensitive marker of infection. Increased shedding of sTNFR1 into 55 serum is predominantly associated with TB rather than HIV infection when examined in subjects categorized into TB only (TB+HIV-), TB and HIV co-infection (TB+HIV+), HIV infection only (TB-HIV+), or neither infection (294). The levels of sTNFR1 are reduced even after anti-tuberculosis treatment (294). Guler et al reported that transgenic mice expressing high serum levels of sTNFR1 exhibited reduced bactericidal activity, undifferentiated granulomas and succumbed to BCG infection (149). Despite the fact that serum levels of sTNFR are also found to be possible markers for infection, unfortunately exposure to mycobacterial antigens alone could also induce sTNFR levels in serum, suggesting that serum analysis might not provide true results. Upon infection with M. tuberculosis it is estimated that about one-third of the world’s population is latently infected and among them the annual risk of reactivation of infection is approximately 0.1%. However, it has been estimated that only 10% of those with latent infection will ever develop active disease in their lifetime (295). The nature of the reactivation of latent infection is explained by the failure of the equilibrium between the host responses and the bacterial growth. Live bacillus within the granuloma can persist for many years as a clinically inactive state (296, 297). Even if the latently infected people pose relatively lower threat compared to the people with clinically active TB disease, however, diagnosis of the latent infection as well as diagnosis of active disease converted from the latent infection is of utmost priority. We therefore, modelled the latent infection and the reactivation of infection in our current study. Results showed that treatment of mice with dexamethasone (DXM), a corticosteroid that suppresses the effector functions of T cells, reactivated the bacterial growth in the lungs from a state of infection, which was experimentally considered latent. Analysis of sTNFRs in BAL and serum indicated that sTNFR levels increased significantly in BAL 2weeks after the DXM treatment (Fig. 3b–c, in Paper I), and this correlated strongly with the bacterial growth in lungs (r 0.9 and r 0.7 for sTNFR1 and sTNFR2, respectively). In contrast, sTNFR levels in serum were not increased and were comparable to the untreated group. Thus, our observation from such reactivation model also suggests that sTNFR level in BAL is a good correlate of active infection. Elevated levels of pro-inflammatory cytokines are a general characteristic of inflammatory responses upon infection. Therefore, TNF production is a prerequisite for the granuloma formation, important for restriction of the mycobacterial growth and dissemination. This supports the fact that neutralization of the TNF activity could lead to mycobacterial growth and we reasoned that elevated levels of sTNFRs resulted in TNF neutralization (149). Of note, TNF in BAL was undetectable in this study using BALB/c mouse strain. Similar observations were made for the BALB/C strain when we compared 56 BALB/c with C57BL/6 mice after i.n. infection with BCG (Paper V, not included in this thesis). Compared to the BALB/c, C57BL/6 mice produced significantly higher amounts of TNF. It is, however, important to acknowledge the fact that the measurement of cytokines or cytokine receptors for instance sTNFRs either in BAL or in serum is not related with the disease specificity. As reported earlier, sTNFR levels are not only increased in mycobacterial infections, but also in many other inflammatory diseases (298-300). Therefore, we reasoned that detection of specific antibodies and cytokines or soluble cytokine receptors could be used as biomarkers for distinguishing active infection from latent infection. Detection of mycobacteria-specific antibodies in serum of TB patients by ELISA is simple, inexpensive and straightforward. Unfortunately, serodiagnosis is still unreliable due to the fact that serodiagnosis alone is unable to differentiate between the active and the exposure to the infection. Antibody responses are mounted during the active infection as well as after being exposed to mycobacterial antigens since this does not require the presence of live bacilli. Results from our study also confirmed this fact that the antigen-specific antibody levels in serum are not true indicators of active infection since the increased antibody levels were observed after treatment of mice with non-replicating BCG or BCG-antigens. In contrast, antigen-specific antibody levels in BAL samples were nearly undetectable after treatment with non-replicating BCG or BCG-antigens. Importantly, we found that antigen specific antibody levels in BAL were increased after infection with live BCG although there was no correlation between the bacterial growth and the antibody levels. This may be expected since the immune system becomes activated by the bacterial presence and starts generating antibody responses, which probably sustained for a certain time. On the other hand, the control of bacterial growth does not necessarily depend on the antibody responses. The cellmediated immune response is most likely involved in the control of mycobacterial infection. Unfortunately, the correlation between active infection and cell-mediated immune responses are also subject to question, because mycobacterial exposure, but not active infection, could induce cell-mediated immune responses. In conclusion, we suggest that single immune parameters are not sufficient to be considered as diagnostic biomarkers of mycobacterial infection even when assessed in the local lung environment, however, this shortcoming could be substantially eliminated by combining two immune parameters such as measurement of TNFRs and antigen-specific antibodies. 57 Paper II Neonatal vaccination with Mycobacterium bovis BCG: potential effects as a priming agent shown in a heterologous prime-boost immunization protocol A new vaccine against TB is urgently needed for the control of the disease. In recent times, there have been significant advances in the development of new TB vaccines particularly in the improvement of the BCG vaccination. The best model for the improvement of BCG vaccination is probably the heterologous prime-boost strategy (258, 301-304). In this protocol, priming with BCG and boosting with either DNA-based vaccine or adjuvanted protein vaccine have been reported to be very efficient in providing protection in animal models of TB. In this study, we evaluated a single mycobacterial protein, HBHA as a BCG boost vaccine. HBHA is one of the most potent mycobacterial antigens tested in murine models for immunization and found to induce protection against M. tuberculosis challenge which is similar to the protection provided by the BCG vaccination (204). We addressed how the neonatal BCG vaccination could contribute as a priming agent to a heterologous primeboost immunization protocol for HBHA. The major findings from this study are a) Neonatal BCG vaccination primed the immune system for native (n) HBHA and therefore upon HBHA boosting, immune responses to HBHA were improved. b) Priming with BCG and boosting with nHBHA (BCG/nHBHA) significantly improved protection against BCG infection independently of the route of nHBHA immunization and the coadministration of adjuvant. c) Immune responses induced after a shorter prime-boost interval decline with time, suggesting that repeated boosting with an optimal prime-boost interval is required. d) Boosting with non-protective form of rHBHA induced protection in the BCG-primed mice. The neonatal immune system is not fully matured and often biased to Th2 type response. The challenge of neonatal vaccination arises due to the poor response of neonates to most vaccines. Neonatal BCG vaccination is one of the few examples that can shift the immune system from a Th2 to Th1 type response (305). Although the BCG-induced immunity wanes with time, BCG vaccination can induce even longer protective immunity than subunit vaccines (212). In order to maintain the BCG-derived immunity, revaccination with BCG has been pursued in different animal models as well as in humans and it revealed that repeated 58 BCG administration did not add benefit rather found to be detrimental (172, 306-309). Revaccination with BCG induces excessive proinflammatory cytokine production and results in lesions formation (306). Prime-boost vaccination strategy has been focused much for the TB vaccine development although it is already in use for humans. Retrospectively, some of the vaccines such as Diphtheria, Tetanus and Pertussis (DPT) are given to the paediatric population three times untill six months of age followed by a final boost between four to six years of age (304). The basic mechanism of this strategy lies on the fact that the first dose of the vaccine primes the immune system and the booster dose with the same vaccine further increases the immune responses and therefore the vaccine-derived immunity lasts long. Recently, it has been discovered that same vaccines given multiple times (called homologous prime-boost vaccination) are less effective than same vaccines delivered in different forms (called heterologous prime-boost vaccination) (304). Heterologous prime-boost technique was first introduced by Shiu-Lok Hu and co-workers in 1992 in the context of HIV vaccine development. Since then many studies were conducted by using this approach for the induction of cellular immunity to a variety of pathogens including M. tuberculosis (170, 310313). In this study, following vaccination with BCG/HBHA combination we observed that BCG vaccination not only primed the immune system to HBHA but also BCG itself acted as an immune adjuvant. We found that introduction of CT adjuvant in nHBHA vaccine did not show a better protection against infection than the nHBHA formulated without CT (Fig. 3 in Paper II). Interestingly, priming with BCG improved protective immune responses even for rHBHA, which was previously reported to be non-protective (204). We observed that lung lymphocytes from the BCG-primed and HBHA-boosted mice produced large amounts of IFNγ upon stimulation with BCG-infected bone-marrow derived macrophages (BMMBCG), a methodological approach suggested to correlate with the level of protection (314, 315). IFNγ levels upon restimulation with HBHA were also induced but were unable to correlate with the level of protection. This perhaps could be explained in two ways: upon BCG infection, BMM present mostly the protective epitope to the lymphocytes from HBHA-immunized mice, which is not the case with HBHA stimulation. In another way, the condition with BMMBCG facilitates antigen presentation in an alternative way, namely cross-presentation. Investigation made by Pramod K. Giri and Jeffrey S. Schorey demonstrated that BMMBCG deliver fully-functional antigens-loaded exosomes and that stimulate both CD4 and CD8 T cells via class I and class II mediated antigen presentation pathways (316). The classical way 59 of assessing the antigen-specific recall immune responses is to restimulate the immune cells with the same antigens used for immunization. Unfortunately, this method does not provide correct information regarding the status of the immunized animals whether protective or nonprotective. Of note, it has been demonstrated previously that, cells isolated from rHBHAimmunized animal were able to produce substantial amount of IFN-γ after in vitro restimulation with rHBHA however, this did not correlate with the protection (204). Similarly, another mycobacterial antigen (27-kDa) used for vaccination has been reported to induce higher levels of IFN-γ but not to offer protection (317). In this regard, many studies have reported the potential use of multifunctional-T cells as immune correlates of protection (257). Even if rHBHA has been found non-protective upon systemic delivery, recent study showed that mucosal delivery of rHBHA offered limited dissemination of mycobacteria from the lungs to the spleen (318). In our study, vaccination with BCG followed by rHBHA delivered either i.n. or s.c. induced a certain level of protection as the bacterial growth was reduced both in the lungs and in the spleen. Presently, we cannot explain the mechanism of protection induced after rHBHA boosting in the BCG-primed animals. Most probably rHBHA acted as a carrier molecule for the protective epitopes generated after BCG vaccination. Induction of protective immune responses in the respiratory compartment is probably the most effective way to prevent pulmonary TB since the respiratory tract is the natural route of M. tuberculosis infection (171, 319, 320). Therefore, our main aim has been centered on the possibility to induce a strong protection against TB based on the lung immunity. Indeed, several studies have already described the potential benefits of mucosal delivery of booster antigens into the BCG-primed animals including the more sensitive guinea-pig models (172, 321). Despite the fact that mucosal delivery of antigens holds the great promise for future vaccination, an implementation of such strategy is under challenge in the case of adjuvantedantigen delivery. Today, there is no secure adjuvant available for human use. Considering this, we primarily aimed to compare i.n. versus s.c. administration of HBHA booster antigen in the BCG-primed mice which showed a comparable level of immune responses after restimulation with BMMBCG and that correlated with the protection (Fig. 2 and 3 in Paper II). Since the s.c. route of immunization was as effective as the i.n. route which is in contrast to the other reports, we examined whether HBHA boosting in the absence of adjuvant could maintain the similar efficacy. Results from our study demonstrated that immune responses and the ability to control bacterial infection were remained unchanged which were comparable to that achieved by adjuvanted-vaccine delivery. 60 Does neonatal BCG vaccination contribute by priming the immune system in advance or offer generalized adjuvant effects? It has been postulated that treatment with the live BCG possesses a more general adjuvant effect and thus able to influence the response to unrelated antigens (322). We continued our investigation to delineate the role of BCG vaccination prior to the boosting with HBHA. Since BCG contains the HBHA antigen it is therefore expected that the immune system could be primed by BCG vaccination. In this study, we also reexamined the BCG-derived adjuvanticity by using ESAT-6, not present in BCG, in a heterologous prime-boost vaccination protocol. We also asked whether the BCG-mediated adjuvanticity is attributable to the status (live or killed) of the bacteria in the BCG-vaccine. Results showed that even if ESAT-6 is not present in BCG, priming with live BCG but not with HK-BCG and boosting with ESAT-6 enhanced ESAT-6-specific recall IFN-γ responses (Fig. 5 in Paper II) indicating that live bacteria could act as an immune adjuvant when injected prior to the antigen. In contrast, both live and HK-BCG were able to increase the recall IFN-γ responses to HBHA (Fig. 5 in Paper II) suggesting that for HBHA, BCG has a dual mode of activity: specific in the case of killed-BCG, and an additional adjuvant effect when given as live bacteria. These results are in agreement with the previously published data where it has been demonstrated that live but not killed BCG is an immunostimulant (323) and due to this property BCG is used as an immunotherapeutic agent against bladder cancer (324). Collectively, our study unveiled how neonatal BCG vaccination could be of importance in the response to a booster antigen whether or not the antigen belongs to the BCG. Additionally, a combination of BCG and HBHA vaccination in the order of BCGpriming and HBHA-boosting offers better immune response and protection regardless of the route of vaccine delivery and adjuvant dependency. Since the immune responses decline with time even following vaccination with BCG/HBHA, thus, repeated boosting with HBHA is necessary for the maintenance of immunological memory. 61 Paper III Influence of maternal gestational treatment with mycobacterial antigens on postnatal immunity in an experimental murine model It is thought that the major constraint for a successful vaccination in the neonates is the immature immune system. In most cases, upon vaccination neonates remain unresponsive to the vaccine antigens or develop Th2 type response. A tendency of developing limited Th1 immune responses could contribute to the severity of infection with intracellular pathogens in the early life. Therefore, to prevent the risk of contracting diseases during the early life, several vaccination strategies are currently in progress. Most recently, early life vaccination, as early as during the fetal life, has been suggested; provided that the fetal immune system could be primed in advance and that neonates become ready for postnatal vaccination. In paper III, we have examined whether the fetal immune system could be primed in advance by gestational treatment with mycobacterial antigens and thus postnatal vaccination could be done efficiently. The major findings from this study are a) In utero exposure to mycobacterial antigens during the 2nd week of pregnancy led to antigen transportation from the mother to the fetus through the placenta. b) Immunization with the same mycobacterial antigen during the postnatal life improved Tcell responses compared to the offspring born to the mother untreated during the gestational period c) Upon challenge with BCG, offspring could control infection better owing to an early exposure of mycobacterial antigen in utero. The placenta is a highly specialized organ that forms an interface between the maternal and the fetal circulation and regulates the transport of materials from the mother to the fetus and wastes in the other direction. Recently, this transportation route has got attention with the probability that mother-derived substances delivered to the fetus could influence immunological activation of the fetal immune system (325-327). However, it is assumed that the placental barrier only allows certain material exchange, which perhaps reduces the chances of the fetal immune system to be exposed to a broad range of substances. Therefore, uncertainty remains in the case of protein antigen transportation through the placenta. On the other hand, it has been shown that maternal immunological experience (transfer of IgG) could 62 be delivered to the fetus through the placenta and this way fetus as well as neonates could be protected (328, 329). Increasing number of evidence suggesting that allergens can be transported through the placenta (330) and the immune system is primed in utero by allergens (326). Therefore, in this study, we addressed whether the fetal immune system is exposed to protein antigens (foreign) and hence could be primed earlier. In this study, we showed that mycobacterial protein antigens were transported through the placenta after administered to the mother at the 2nd week of pregnancy. Fluorescent quantum dot conjugated with protein antigens were injected to the mother and several hours postinjection fluorescence signals were detected in the whole fetus and placenta samples. A time point of gestational period could be important for the maturation of placenta as well as for the development of fetal cells. In mice, two weeks of gestational period seem to be enough for the maturation of placenta. We have investigated the effects of antigen transfer from the mother to the fetus on the immune responses generated in the offspring. In order to examine this antigen-specific effect we have immunized offspring mice with the same antigens used for maternal treatment. As previously stated, in addition to transplacental antigen transportation, antigens could also be transported via milk. We examined this effect by including a group of newborn mice born to the treated mother and nursed by the untreated mother. Antigen-specific T cell and B cell responses in the offspring mice were examined. For T cell activation, lung lymphocytes from the immunized mice were restimulated in vitro with the antigens and IFN-γ responses were determined. For B cell responses, antigen specific IgM and IgG antibody levels were measured in sera of immunized mice. We observed that antigen-specific T-ell responses (IFNγ levels) were significantly higher in the mice born to the treated mother than the offspring born to the untreated control mother (Table 1, in Paper III). Similarly, significantly higher levels of T-cell responses were observed in the offspring mice born to the treated mother but nursed by the untreated foster mother. Antigen-specific antibody responses were remained unchanged in the offspring mice born to the treated mother as compared with their control littermates. These results indicated that gestational treatment with mycobacterial antigens resulted in priming of the fetal immune system and that enhanced the T-cell responses following postnatal immunization. We also examined antibody responses in the pregnant mother post-treatment with antigen and found that there was not any detectable antibody response in the serum. Since the gestational treatment was performed in the absence of adjuvant this perhaps explained why the treated mother had no detectable antibody response. Thus, the results from this study indicated that there was no influence of maternal antibody response on the T cell responses in the offspring. 63 Why was the T cell compartment but not the B cell compartment sensitised following gestational treatment? An analysis of murine T- and B-cell ontogeny revealed that B-cell development is delayed compared to the T-cell development. Fetal thymocytes are able to proliferate in response to T-cell mitogens by day 17 of gestation (331) and also a few matured CD4 and CD8 T cells can be found immediately before birth. In contrast, the B-cell development starts after birth. This species-specific T- and B-cell ontogeny might explain the reason why we have seen the activation only in the T-cell compartment after gestational treatment. Compared to the mouse T- and B-cell ontogeny, in humans functional T and B cells are found before birth (331). An increasing number of studies in human models proposed that fetal or neonatal immune responses are increased by the prenatal priming (199, 332) and this might occur due to the transfer of antigens through the placenta. However, in those studies, there was no direct evidence showing that antigens were transported through the placenta. Our current study has provided evidence for the placental-antigen transportation and its effects on postnatal immune responses. It is, however, not clear how fetal T cells were primed following gestational treatment with antigens. Priming of the T cells requires antigen presentation by APCs. Therefore, the APCs in utero should have attained certain level of maturity to present antigen to the T cells. Very little is known about the phenotype and functional development of APCs during the fetal life. In humans, it has been shown that the percentage of MHC Class II-positive fetal monocytes was increased over the gestational period although it was lower compared to the adult monocytes (185). Moreover, the percentage of CD40+ or CD86+ fetal/neonatal monocytes was comparable to the adult (185). The BCG vaccine against adult pulmonary TB induces partial protection but its efficacy is more satisfactory against disseminated forms of TB. Thus, the BCG vaccination is still recommended in many countries around the world where the risk of contracting infection is high. In general, the BCG vaccine is safe for humans with the exception of a few reports where disseminated BCG disease was detected post BCG vaccination (333). Disseminated BCG disease is very uncommon and might be seen in immunocompromised infants or adult individuals with HIV infection. This poses a risk against the continuation of BCG vaccination in immunocompromised individuals. Thus, the findings from this current study might be useful for the development of a future TB vaccine targeting a particular group of people, who are at greater risk of receiving the BCG vaccine. 64 Paper IV Immunization with mycobacterial antigens: a role for innate immunity in antigen presentation TLR2 has been identified as an important mediator of macrophage activation in response to M. tuberculosis (334). Three structurally different components of mycobacterial cell wall fractions enriched with LAM, mycolylarabionogalactan-peptidoglycan complex or total lipids induce TNF production upon recognition by macrophages via TLR2 (334). TNF production is important for granuloma formation and induction of protective immune responses (334). In the absence of TLR2, macrophages have decreased ability to produce TNF in response to mycobacterial infection and animals are susceptible to mycobacterial infection (335, 336). In Paper IV, we aimed at investigating the role of TLR2 for the induction of antigen-specific immune responses upon immunization. We immunized wild-type (WT) and TLR2-/- mice with mycobacterial 19kDa (TLR2-agonist) or Ag85A (irrelevant for TLR2). We measured antigen-specific humoral and cellular immune responses. We also examined whether TLR2 is necessary to activate APCs and to promote antigen presentation in vitro. The major findings from this study are a) Antigen-specific antibody responses were comparable between the WT and TLR2-/- mice. b) Immunization with 19kDa induced more Th1 type response as reflected by the increased levels of IgG2a in serum. c) Recall IFN-γ responses to 19kDa were significantly lower in the TLR2-/- mice compared to the WT-type mice. d) Spleen cells from TLR2-/- mice secreted comparable levels of IFN-γ in response to BMMBCG. Our data from this study showed that antigen-specific antibody responses in serum were enhanced both in the WT and TLR2-/- mice (Table 1, Paper IV). 19kDa is a culture filtrate protein of M. tuberculosis and by virtue of its potent immunostimulatory properties, immunization with 19kDa induced potent humoral and cellular immune responses, which have also been described previously (336, 337). Purified 19kDa antigen induces proinflammatory cytokine IL-12 production in the macrophages and could influence the development of Th1 type response in the host (338). Consistent with this notion, we found that mice immunized with 19kDa had more Th1 type response than mice that received Ag85A. The immune responses induced in the TLR2-/- mice raised the question about how 65 the 19kDa antigen was taken up by the cells and how the immune system was primed in the absence of TLR2. There has been data indicating that 19kDa lipoprotein of M. tuberculosis functions as a major adhesin, interacts with MR and promotes phagocytosis of mycobacteria (339). The 19kDa antigen that we used for immunization was a purified recombinant protein without posttranslational modifications, hence we speculate that in the absence of TLR2, 19kDa antigen can be internalized by other possible receptor-mediated endocytic mechanisms, which include membrane immunoglobulin or Fc-receptor-mediated interaction, micropinocytosis or macropinocytosis (340). We attempted to determine the cellular immune responses by restimulating spleen cells from the immunized mice with the antigens. Upon in vitro restimulation primed cells become activated and secret IFN-γ in the culture supernatants. We observed that cells from the Ag85A-immunized mice produced comparable levels of IFN-γ independently of the type of mouse strains. By contrast, a noticeable difference was found in the case of the 19kDaimmunized mice. In the absence of TLR2, cells produced less IFN-γ, suggesting two possibilities: either the T cells were not properly primed in vivo or the antigen presentation in vitro was not sufficient. We addressed these two questions in the subsequent in vitro experiments by coculturing spleen cells from TLR2-/- mice with BMM pulsed with antigen (BMMAg) from WT mice and vice versa. Our data from this experiments showed that both spleen cells and BMM from TLR2-/- mice were functionally active when cocultured with BMM and spleen cells from WT-type mice respectively. These results indicated that probably the immunological synapse between the T cells and the BMM was not sufficiently formed in the absence of TLR2. If this is true, the T-cell receptor (TCR) and MHC-peptide complex was not formed or the necessary costimulatory signals were not generated. However, the former might not be possible since the IFN-γ responses were not completely abolished in the absence of TLR2. Thus, we examined the expression of costimulatory molecules in the BMM upon activation with 19kDa and the data revealed that CD86 costimulatory molecules were not upregulated in the TLR2-/- BMM, indicating that TLR2-dependent activation was necessary. By contrast, TLR2-dependent activation of BMM was not seen when BMM were pulsed with BCG (BMMBCG). Surface expression of CD86 and CD80 was increased in both WT-type TLR2-/- BMMBCG. Hence, spleen cells from TLR2-/- mice were activated in the presence of TLR2-/- BMMBCG. These results suggested that APCs from TLR2-/- mice could be differentially activated when pulsed with BCG instead of 19kDa antigen. 66 Although the 19kDa antigen is a potent Th1-promoting agent, several in vitro investigations have shown that prolonged exposure to the 19kDa antigen through TLR2 inhibited antigen processing by reducing MHC II expression (341, 342) and also interfered with IFN-γ-induced mycobacterial killing (343). Likewise, it has been shown in vivo that immune responses were modulated by 19kDa after immunization with rBCG overexpressing the 19kDa antigen (rBCG19N) (338, 344). Upon overexpression of 19kDa, responses to BCG-antigens were polarised towards Th2 type (338). In contrast, responses to 19kDa antigen remained Th1 type (338). Consistent with the previous observations, we found Th1dominated humoral and cellular immune responses following immunization with the 19kDa antigen. In contrast to the in vitro data that support the inhibitory role of the 19kDa antigen, we however, did not observe any inhibitory function of 19kDa in the BMM pulsed with 19kDa. Therefore, cell surface expression of MHC II, CD86 and CD80 was not inhibited. Since 19kDa is a TLR2 ligand, we rather found TLR2-dependent activation of BMM after pulsing with 19kDa. Together, our results from this study suggested that even a lower level, immune responses could be generated in the absence of TLR2. TLR2-mediated innate responses were important for the presentation of the 19kDa antigen and activation of T cells. Thus, in the absence of TLR2, 19kDa-specific responses were substantially compromised. 67 Concluding remarks and future perspectives The current studies have focused on two issues; improvement of the diagnostic procedures and the development of better vaccines which are likely the key components in the overall strategy for the control of TB. We reasoned that understanding of the local (respiratory mucosal) immune responses would be more rational since the respiratory tract is the natural route of M. tuberculosis infection. In Paper I, we have shown that the detection of cytokines or cytokine receptors together with antigen-specific antibodies IgG or IgA in the respiratory tract but not in the serum distinguishes active infection from latent infection or exposure with mycobacterial antigens. Cytokines or cytokine receptors correlate better with active infection and specific antibody responses are needed for the identification of the causative organisms. Based on the findings from Paper I we envisage that it will be of clinical importance to examine these immunological parameters in patient with active or latent infections. In humans, obtaining bronchoalveolar lavage samples represents a challenge to the implication of this strategy. Therefore, one approach to meeting this challenge and clinical entropy might be to use human saliva or sputum samples for the detection of immunological biomarkers. To ameliorate TB vaccination strategies, in Paper II, we attempted to understand how the BCG vaccination works after neonatal administration and whether the immune responses could be enhanced by boosting with mycobacterial HBHA. The results indicate that BCG vaccination has a dual role: BCG-vaccination primes the neonatal immune system for HBHA and thus boosting with HBHA further improves immune responses and protection in the respiratory tract; BCG-vaccination has a generalized adjuvant effects to BCG-related or unrelated antigens. Priming with BCG followed by boosting with HBHA improves the immune responses and protection independently of the route of vaccination and the use of adjuvant. This heterologous prime-boost protocol shows that the magnitude of the immune responses drops with time, suggesting that repeated booster immunizations are needed to maintain the memory. Since the neonatal BCG-vaccination is still recommended for the place where the risk of childhood TB is high, it is logical to use the BCG as a priming vaccine. In the future studies using more sensitive animal models, a comprehensive analysis is required to further assess the importance of the BCG as a priming vaccine. Essentially, the route of vaccination and the need of adjuvants are needed to be re-examined in the course of generating immune responses and protection in the lungs. In Paper III, we sought to examine whether neonatal vaccination with mycobacterial antigens could be improved by prenatal priming. Summarizing the results from Paper III, we conclude 68 that maternal gestational treatment with mycobacterial antigens primes the fetal immune system; therefore, postnatal immunization with the same antigens enhanced immune responses and protection in the offspring mice. We provide evidence that mycobacterial antigens can be transported from the mother to the fetus through the placenta. In a future perspective, it would be of great importance to get an in-depth knowledge on the maternalfetal immunological interactions in relation to the antigen presentation to the T cells and generation of memory T cells during the fetal stage. Species differences are obvious regarding the ontogeny of immune cells, thus one would have to examine whether placental-antigen transportation could prime the fetal immune system in humans. It would be of great interest to examine the influence of maternal-immune mediators on the prenatal priming and thus to unfold immune-regulatory mechanisms in connection with the neonatal unresponsiveness. It is likely that the neonatal BCG vaccination in the immunocompromised individuals results in BCG-itis (regional disease) or BCG-osis (disseminated disease), therefore the strategy with the prenatal priming with mycobacterial antigens holds a great promise particularly for the new generation of TB vaccines. In Paper IV, we addressed the role of innate immunity in the course of generating adaptive immune responses. In this preliminary manuscript, we conclude that immunization with mycobacterial antigens enhances antigen-specific humoral and cellular immune responses even in the absence of TLR2. Memory T cells from TLR2-/- mice are not sufficiently activated upon in vitro restimulation with 19kDa antigen presumably it requires TLR2dependent activation of APCs. Formation of TCR and MHC-peptide complex followed by the expression of necessary costimulatory molecules might be required for the generation of immunological synapse. Future studies should investigate how innate immune responses could contribute to the formation of immunological synapse between the antigen-specific T cells and the APCs. 69 Acknowledgements This study was carried out at the Department of Immunology, Stockholm University. I am grateful to all who were involved in these studies, in particular my supervisor Prof. Carmen Fernández for her strong support from the first day in Sweden. I thank Carmen very much for opening a PhD position for me and assisted me in my growth scientifically over the past years. I thank Prof. Marita Troye-Blomberg, Prof. Klavs Berzins, Prof. Eva Severinson and Associate Prof. Eva Sverrenmark for all the important suggestions towards improving the quality of the work. Apart from the scientific discussion you all have given a nice friendly environment to the immunology department and I feel proud of that. My special thanks to a former PhD student John Arko-Mensah, who worked in the TB project with me and we shared first authorship in some articles. I appreciate John very much for his relentless cooperation in the laboratory. I thank Olga Flores who is currently a PhD student for providing me help whenever I asked for. I also thank Irene Roman for her active participation and strong will towards achieving results. I am grateful to Eshter Julián, a former postdoc in our group, for her excellent care at the beginning of my degree project. I appreciate Eva Nygren and all the staffs at the animal house for their important support in the animal house. Especially, I got remarkable help from Eva Ngren with all the breeding experiments. I thank Manijeh Avedi for helping me with FACS antibodies. I thank Magaretha Hagstedt, Ann Sjörlund, Gelana Yadeta, Anna Leena for their invaluable assistance. I will never forget your contribution. I would like to extend my warmest thanks to Ebba, Maria, Pablo, charles and Shahid. You were as nice to me as you are always. I found you extremely cooperative. Thanks to all past and present colleagues at the department especially Shanie, Yvonne and Salah for sharing the knowledge of Flow cytometry. Thanks also to katharina, Stefania, Anna, Petra, Halima, Manijeh, Lisa, Nora, Ylva, Nancy, Khosro, Jacqueline, Gishanti, Amre, Nnaemeka, Jacob, Gudrun, Sandra, Christian, Ulrika, Magdi, and Camilla for the nice discussion at the department. I appreciate beyond words the families who over the years supported me for everything. A special thanks to my wife for her patience and constant encouragement. Thank you Ilma, my little daughter, with a smile always, a special gift from the Almighty. You have got an unbelievable understanding which helped your pappa working at home. You never annoyed me while I was writing rather brought my hand to the computer whenever you saw me resting. 70 My special thanks to Qazi Khaleda Rahman who actually paved the way for us in Sweden for higher study. I thank Apu Bhai who is very nice person that I ever found. Thanks to Ariane for your cute and lovely gesture that gave me immense pleasure. I thank Dr. Atikul Islam and Dr. Zarina Kabir for helping my family on several occasions. I owe a debt of gratitude to my parents and sisters for their continuing support, grounding and love. These studies were financially supported by grants from Hja¨rt-Lungfonden, European Commission, FP6 and FP7, LSHP-CT-2005 018736 and HOMITB200732 respectively. 71 References 1. World Health Organization WHO Report 2007. Global Tuberculosis Control, Surveillance, Planning, Finanacing: Geneva, WHO, 2007, 227pp. 2. Raviglione MC. The TB epidemic from 1992 to 2002. Tuberculosis (Edinb) 2003;83:4-14. 3. Mitnick CD, Shin SS, Seung KJ, et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med 2008;359:563-74. 4. Kaufmann SH. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol 2001;1:20-30. 5. Fine PE. The BCG story: lessons from the past and implications for the future. Rev Infect Dis 1989;11:S353-9. 6. Collins HL, Kaufmann SH. Prospects for better tuberculosis vaccines. Lancet Infect Dis 2001;1:218. 7. Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe 2008;3:399-407. 8. Makinoshima H, Glickman MS. Regulation of Mycobacterium tuberculosis cell envelope composition and virulence by intramembrane proteolysis. Nature 2005;436:406-9. 9. Roth MG. Phosphoinositides in constitutive membrane traffic. Physiol Rev 2004 ;84:699-730. 10. Vergne I, Chua J, Deretic V. Mycobacterium tuberculosis phagosome maturation arrest: selective targeting of PI3P-dependent membrane trafficking. Traffic 2003;4:600-6. 11. Vergne I, Chua J, Deretic V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J Exp Med 2003;198:653-9. 12. Ferrari G, Langen H., Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 1999;97:435–447. 13. Gatfield J, Albrecht I., Zanolari B, et al. Association of the Leukocyte Plasma Membrane with the Actin Cytoskeleton through Coiled Coil-mediated Trimeric Coronin 1 Molecules Mol. Biol. Cell 2005;16:2786–98. 14. Cosma CL, Sherman DR, Ramakrishnan L. The secret lives of the pathogenic mycobacteria. Annu Rev Microbiol 2003;57:641-76. 15. Flynn JL, Chan J. Immune evasion by Mycobacterium tuberculosis: living with the enemy. Curr Opin Immunol 2003;15:450-5. 16. Dannenberg AM Jr. Macrophage turnover, division and activation within developing, peak and "healed" tuberculous lesions produced in rabbits by BCG. Tuberculosis (Edinb) 2003;83:251-60. 17. Dheda K, Booth H, Huggett JF, et al. Lung remodeling in pulmonary tuberculosis. J Infect Dis 2005;192:1201-9. 18. Bartlett JG. Tuberculosis and HIV infection: partners in human tragedy. J Infect Dis 2007 ;196:S124-5. 72 19. Sousa AO, Salem JI, Lee FK, et al. An epidemic of tuberculosis with a high rate of tuberculin anergy among a population previously unexposed to tuberculosis, the Yanomami Indians of the Brazilian Amazon. Proc Natl Acad Sci U S A 1997;94:13227-32. 20. Kramnik I, Dietrich WF, Demant P, et al. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 2000;97:8560-5. 21. Hill AV. The immunogenetics of human infectious diseases. Annu Rev Immunol 1998;16:593617. 22. Stead WW. Genetics and resistance to tuberculosis. Could resistance be enhanced by genetic engineering? Ann Intern Med 1992;116:937-41. 23. Buschman E, Apt AS, Nickonenko BV, et al. Genetic aspects of innate resistance and acquired immunity to mycobacteria in inbred mice. Springer Semin Immunopathol 1988;10:319-36. 24. Lynch CJ, Pierce-Chase CH, Dubos R. A genetic study of susceptibility to experimental tuberculosis in mice infected with mammalian tubercle bacilli. J Exp Med 1965;121:1051-70. 25. Medina E, North RJ. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology 1998;93:270-4. 26. Nikonenko BV, Apt AS, Mezhlumova MB, et al. Influence of the mouse Bcg, Tbc-1 and xid genes on resistance and immune responses to tuberculosis infection and efficacy of bacille Calmette-Guérin (BCG) vaccination. Clin Exp Immunol 1996;104:37-43. 27. Fortin A, Abel L, Casanova JL, et al. Host genetics of mycobacterial diseases in mice and men: forward genetic studies of BCG-osis and tuberculosis. Annu Rev Genomics Hum Genet 2007;8:16392. 28. Schnappinger D, Schoolnik GK, Ehrt S. Expression profiling of host pathogen interactions: how Mycobacterium tuberculosis and the macrophage adapt to one another. Microbes Infect 2006;8:113240. 29. Marquis JF, Nantel A, LaCourse R, et al. Fibrotic response as a distinguishing feature of resistance and susceptibility to pulmonary infection with Mycobacterium tuberculosis in mice. Infect Immun 2008;76:78-88. 30. Chackerian AA, Behar SM. Susceptibility to Mycobacterium tuberculosis: lessons from inbred strains of mice. Tuberculosis (Edinb) 2003;83:279-85. 31. Hoal EG, Lewis LA, Jamieson SE, et al. SLC11A1 (NRAMP1) but not SLC11A2 (NRAMP2) polymorphisms are associated with susceptibility to tuberculosis in a high-incidence community in South Africa. Int J Tuberc Lung Dis 2004;8:1464-71. 32. Malik S, Abel L, Tooker H, et al. Alleles of the NRAMP1 gene are risk factors for pediatric tuberculosis disease. Proc Natl Acad Sci U S A 2005;102:12183-8. 33. Li HT, Zhang TT, Zhou YQ, et al. SLC11A1 (formerly NRAMP1) gene polymorphisms and tuberculosis susceptibility: a meta-analysis. Int J Tuberc Lung Dis 2006;10:3-12. 34. Delgado JC, Baena A, Thim S, et al. Aspartic acid homozygosity at codon 57 of HLA-DQ beta is associated with susceptibility to pulmonary tuberculosis in Cambodia. J Immunol 2006;176:1090-7. 73 35. Barreiro LB, Neyrolles O, Babb CL, et al. Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med 2006;3:e20. 36. Berrington WR, Hawn TR. Mycobacterium tuberculosis, macrophages, and the innate immune response: does common variation matter? Immunol Rev 2007;219:167-86. 37. Schlesinger LS, Bellinger-Kawahara CG, Payne NR, et al. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement C3. J Immunol 1990;144:2771. 38. Senbagavalli P, Geetha ST, Karunakaran K, et al. Reduced erythrocyte CR1 levels in patients with pulmonary tuberculosis is an acquired phenomenon. Clin Immunol 2008 ;128:109-15. 39. Hu C, Mayadas-Norton T, Tanaka K, et al. Mycobacterium tuberculosis infection in complement receptor 3-deficient mice. J Immunol 2000;165:2596-602 40. Tailleux L, Pham-Thi N, Bergeron-Lafaurie A, et al. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med 2005;2:e381. 41. Geijtenbeek TB, Van Vliet SJ, Koppel EA, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med 2003;197:7–17. 42. Maeda N, Nigou J, Herrmann JL, et al. The cell surface receptor DCSIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J Biol Chem 2003;278:5513–5516. 43. Tailleux L, Schwartz O, Herrmann JL, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med 2003;197:121–127. 44. Gringhuis SI, den Dunnen J, Litjens M, et al. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 2007;26:605-16. 45. Kawai T, Akira S. TLR signaling. Cell Death Differ 2006;13:816-25. 46. Thoma-Uszynski S, Kiertscher SM, Ochoa MT, et al. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J Immunol 2000;165:3804-3810. 47. Hertz CJ, Kiertscher SM, Godowski PJ, et al. Microbial lipopeptides stimulate dendritic cell maturation via toll-like receptor 2. J Immunol 2001;166:2444-50. 48. Heldwein KA, Fenton MJ. The role of Toll-like receptors in immunity against mycobacterial infection. Microbes Infect 2002;4:937-44. 49. Quesniaux V, Fremond C, Jacobs M, et al. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect 2004;6:946-59. 50. Means TK, Lien E, Yoshimura A, et al. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol 1999 15;163:674855. 51. Fremond CM, Nicolle DM, Torres DS, et al. Control of Mycobacterium bovis BCG infection with increased inflammation in TLR4-deficient mice. Microbes Infect 2003 ;5:1070-81. 74 52. Rook GA, Steele J, Fraher L, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology 1986 ;57:159-63. 53. Liu PT, Modlin RL. Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol 2008;20:371-6. 54. Chocano-Bedoya P, Ronnenberg AG. Vitamin D and tuberculosis. Nutr Rev 2009 ;67:289-93. 55. Ferwerda G, Girardin SE, Kullberg BJ, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog 2005 ;1:279-85. 56. Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 2005;307:731-4. 57. Gandotra S, Jang S, Murray PJ, et al. Nucleotide-binding oligomerization domain protein 2deficient mice control infection with Mycobacterium tuberculosis. Infect Immun 2007 ;75:5127-34. 58. Armstrong JA, Hart PD. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med 1975;142:1-16. 59. Le Cabec V, Cols C, Maridonneau-Parini I. Nonopsonic phagocytosis of zymosan and Mycobacterium kansasii by CR3 (CD11b/CD18) involves distinct molecular determinants and is or is not coupled with NADPH oxidase activation. Infect Immun 2000;68:4736-45. 60. Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 1994;263: 678-81. 61. Kang PB, Azad AK, Torrelles JB, et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med 2005;202:987-99. 62. Pompei L, Jang S, Zamlynny B, et al. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. J Immunol 2007;178:51929. 63. Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 2009;27:393422. 64. Xia W, Pinto C, Kradin R.. The antigen-presenting activities of Ia+ dendritic cells shift dynamically from lung to lymph node after an airway challenge with soluble antigen. J Exp Med 1995;181:1275–83. 65. Holt P, Haining S, Nelson D, et al. Origin and steady-state turnover of class II MHCbearing dendritic cells in the epithelium of the conducting airways. J Immunol 1994; 153:256–61. 66. Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun 1996;64:1400–06. 67. Menozzi FD, Rouse JH, Alavi M, et al. Identification of a heparin-binding hemagglutinin present in mycobacteria. J Exp Med 1996;184:993-1001. 68. Saiga H, Nishimura J, Kuwata H, et al. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J Immunol 2008;181:8521-7. 75 69. Debbabi H, Ghosh S, Kamath AB, et al. Primary type II alveolar epithelial cells present microbial antigens to antigen-specific CD4+ T cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L274-9. 70. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol 2006;8:1687-96. 71. Tan BH, Meinken C, Bastian M, et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol 2006;177:1864-71. 72. Pedrosa J, Saunders BM, Appelberg R, et al. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun 2000 ;68:577-83. 73. Fulton SA, Reba SM, Martin TD, et al. Neutrophil-mediated mycobacteriocidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect Immun 2002;70:5322-7. 74. Van Gisbergen KP, Sanchez-Hernandez M, Geijtenbeek TB, et al. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DCSIGN. J Exp Med 2005;201:1281-92. 75. Megiovanni AM, Sanchez F, Robledo-Sarmiento M, et al. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J Leukoc Biol 2006;79:977-88. 76. Eruslanov EB, Lyadova IV, Kondratieva TK, et al. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun 2005;73:1744-53. 77. Junqueira-Kipnis AP, Kipnis A, Jamieson A, et al. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol 2003;171:6039-45. 78. Dhiman R, Indramohan M, Barnes PF, et al. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol 2009;183:6639-45. 79. Feng CG, Kaviratne M, Rothfuchs AG, et al. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J Immunol 2006;177:7086-93. 80. Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev 1998;11:514-32. 81. Glatman-Freedman A. The role of antibody-mediated immunity in defense against Mycobacterium tuberculosis: advances toward a novel vaccine strategy. Tuberculosis (Edinb) 2006;86:191-7. 82. Raviglione MC, Snider DE Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 1995;273:220-6. 83. Barnes PF, Bloch AB, Davidson PT, et al. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644-50. 84. Hopewell PC. Impact of human immunodeficiency virus infection on the epidemiology, clinical features, management, and control of tuberculosis. Clin Infect Dis 1992;15:540-7. 85. Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med 1999;340:367-73. 76 86. Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol 1987;138:293-8. 87. Mogues T, Goodrich ME, Ryan L, et al. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med 2001;193:271-80. 88. Orme IM, Collins FM. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med 1983;158:74-83. 89. Caruso AM, Serbina N, Klein E, et al. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol 1999;162:5407-16. 90. Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev 1995;146:57-79. 91. Flynn JL, Goldstein MM, Triebold KJ, et al. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 1992;89:12013-7. 92. Canaday DH, Wilkinson RJ, Li Q, et al. CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J Immunol 2001;167:2734-42. 93. Stenger S, Hanson DA, Teitelbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998;282:121-5. 94. Stegelmann F, Bastian M, Swoboda K, et al. Coordinate expression of CC chemokine ligand 5, granulysin, and perforin in CD8+ T cells provides a host defense mechanism against Mycobacterium tuberculosis. J Immunol 2005;175:7474-83. 95. Cooper AM, D'Souza C, Frank AA, et al. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun 1997;65:1317-20. 96. Tascon RE, Stavropoulos E, Lukacs KV, et al. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun 1998;66:830-4. 97. Boom WH. New TB vaccines: is there a requirement for CD8 T cells? J Clin Invest 2007 ;117:2092-4. 98. Lewinsohn DM, Alderson MR, Briden AL, et al. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J Exp Med 1998 ;187:1633-40. 99. Lewinsohn DM, Briden AL, Reed SG, et al. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J Immunol 2000;165:925-30. 100. Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145-73. 101. Lyakh L, Trinchieri G, Provezza L, et al. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev 2008;226:112-31. 77 102. Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 2007;13:139–145. 103. Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007;8:369-77. 104. Schild H, Mavaddat N, Litzenberger C, et al. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell.1994;76:29–37. 105. Tanaka Y, Morita CT, Tanaka Y, et al. Natural and synthetic nonpeptide antigens recognized by human gamma delta T cells. Nature 1995;375:155–58. 106. Tanaka Y, Sano S, Nieves E, et al. Nonpeptide ligands for human gamma delta T cells. Proc. Natl. Acad. Sci. USA 1994;91:8175-79. 107. Holtmeier W, Kabelitz D. γδ T cells link innate and adaptive immune responses. Chem Immunol Allergy 2005;86:151-183. 108. Lahn M. The role of gammadelta T cells in the airways. J Mol Med 2000;78:409-25. 109. Dieli F, Blomberg TM, Ivanyi J, et al. Vgamma9/Vdelta2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur J Immunol 2000;30:1512–1519. 110. Tsukaguchi K, Balaji KN, Boom WH. CD4+ alpha beta T-cell and gamma delta T-cell responses to Mycobacterium tuberculosis. Similarities and differences in Ag recognition, cytotoxic effector function, and cytokine production. J Immunol 1995;154:1786–96. 111. De La Barrera SS, Finiasz M, Frias A, et al. Specific lytic activity against mycobacterial antigens is inversely correlated with the severity of tuberculosis. Clin Exp Immunol 2003 ;132:450-61. 112. D'Souza CD, Cooper AM, Frank AA, et al. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol 1997;158:1217-21. 113. Sutton CE, Lalor SJ, Sweeney CM, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009;31:331-41. 114. Roark CL, Simonian PL, Fontenot AP, et al. gammadelta T cells: an important source of IL-17. Curr Opin Immunol 2008;20:353-7. 115. Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science 2005;309:264-8. 116. Moser B, Brandes M. Gammadelta T cells: an alternative type of professional APC. Trends Immunol 2006;27:112-8. 117. Moser B, Eberl M. gammadelta T cells: novel initiators of adaptive immunity. Immunol Rev 2007;215:89-102. 118. Suffia IJ, Reckling SK, Piccirillo CA, et al. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med 2006;203:777-88. 119. Ribeiro-Rodrigues R, Resende Co T, Rojas R, et al. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol 2006 ;144:25-34. 78 120. Green EA, Gorelik L, McGregor CM, et al. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci U S A 2003;100:10878-83. 121. Guyot-Revol V, Innes JA, Hackforth S, et al. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med 2006;173:803-10. 122. Williams A, Reljic R, Naylor I, et al. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology 2004;111:328-33. 123. Reljic R, Clark SO, Williams A, et al. Intranasal IFNgamma extends passive IgA antibody protection of mice against Mycobacterium tuberculosis lung infection. Clin Exp Immunol 2006;143:467-73. 124. Vordermeier HM, Venkataprasad N, Harris DP, et al. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol 1996;106:312-6. 125. Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol 2007;178:722234. 126. de Vallière S, Abate G, Blazevic A, et al. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun 2005;73:6711-20. 127. Rodríguez A, Tjärnlund A, Ivanji J, et al. Role of IgA in the defense against respiratory infections IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine 2005;23:2565-72. 128. Teitelbaum R, Glatman-Freedman A, Chen B, et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A 1998;95:15688-93. 129. Hamasur B, Haile M, Pawlowski A, et al. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab') fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol 2004;138:30-8. 130. Pethe K, Alonso S, Biet F, et al. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 2001;412:190-4. 131. Maglione PJ, Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur J Immunol 2009;39:676-86. 132. Maglione PJ, Xu J, Casadevall A, et al. Fc gamma receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J Immunol 2008;180:3329-38. 133. Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683-765. 134. Junqueira-Kipnis AP, Kipnis A, Henao Tamayo M, et al. Interleukin-10 production by lung macrophages in CBA xid mutant mice infected with Mycobacterium tuberculosis. Immunology 2005;115:246-52. 135. Cooper AM, Dalton DK, Stewart TA, et al. Disseminated tuberculosis in interferon gamma genedisrupted mice. J Exp Med 19931;178:2243-7. 79 136. Dalton DK, Pitts-Meek S, Keshav S, et al. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 1993;259:1739-42. 137. Flynn JL, Chan J, Triebold KJ, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 1993;178:2249-54. 138. Cooper AM, Magram J, Ferrante J, et al. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med 1997;186:39-45. 139. Jouanguy E, Döffinger R, Dupuis S, et al. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol 1999;11:346-51. 140. Alcaïs A, Fieschi C, Abel L, et al. Tuberculosis in children and adults: two distinct genetic diseases. J Exp Med 2005;202:1617-21. 141. Cooper AM, Roberts AD, Rhoades ER, et al. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology 1995;84:423-32. 142. Cooper AM, Kipnis A, Turner J, et al. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol 2002;168:1322-7. 143. Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev 2008;226:191-204. 144. Chan J, Xing Y, Magliozzo RS, et al. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med 1992;175:1111–22. 145. Chan J, Tanaka K, Carroll D, et al. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun 1995;63:736–40. 146. Sabatini F, Silvestri M, Sale R, et al. Fibroblast-eosinophil interaction: modulation of adhesion molecules expression and chemokine release by human fetal lung fibroblasts in response to IL-4 and TNF-alpha. Immunol Lett 2002;84:173-8. 147. Bean AGD, Roach DR, Briscoe H, et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection which is not compensated for by lymphotoxin. J Immunol 1999;162:3504–11. 148. Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-α is required in the protective immune response against M. tuberculosis in mice. Immunity 1995;2:561–72. 149. Guler R, Olleros ML, Vesin D, et al. Differential effects of total and partial neutralization of tumor necrosis factor on cell-mediated immunity to Mycobacterium bovis BCG infection. Infect Immun 2005;73:3668-76. 150. Rook GA. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med 2007;7:327-37. 151. Seah GT, Scott GM, Rook GA. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis 2000;181:385-9. 152. van Crevel R, Karyadi E, Preyers F, et al. Increased production of interleukin 4 by CD4+ and CD8+ T cells from patients with tuberculosis is related to the presence of pulmonary cavities. J Infect Dis 2000;181:1194-7. 80 153. Bogdan C, Vodovotz Y, Paik J, et al. Mechanism of suppression of nitric oxide synthase expression by interleukin-4 in primary mouse macrophages. J Leukoc Biol 1994;55:227–33. 154. Demissie A, Abebe M, Aseffa A, et al. Healthy individuals that control a latent infection with Mycobacterium tuberculosis express high levels of Th1 cytokines and the IL-4 antagonist IL-4delta2. J Immunol 2004;172:6938–43. 155. Fletcher HA, Owiafe P, Jeffries D, et al. Increased expression of mRNA encoding interleukin (IL)-4 and its splice variant IL-4delta2 in cells from contacts of Mycobacterium tuberculosis, in the absence of in vitro stimulation. Immunology 2004;112:669–73. 156. Algood HM, Chan J, Flynn JL. Chemokines and tuberculosis. Cytokine Growth Factor Rev 2003;14:467-77. 157. Sadek MI, Sada E, Toossi Z, et al. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol. 1998;19:513-21. 158. Kurashima K, Mukaida N, Fujimura M, et al. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am J Respir Crit Care Med 1997 ;155:1474-7. 159. Scott HM, Flynn JL. Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: influence of dose on disease progression. Infect Immun 2002;70:5946-54. 160. Peters W, Scott HM, Chambers HF, et al. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2001;98:7958-63. 161. Rutledge BJ, Rayburn H, Rosenberg R, et al. High level monocyte chemoattractant protein-1 expression in transgenic mice increases their susceptibility to intracellular pathogens. J Immunol 1995;155:4838-43. 162. Di Liberto D, Locati M, Caccamo N, et al. Role of the chemokine decoy receptor D6 in balancing inflammation, immune activation, and antimicrobial resistance in Mycobacterium tuberculosis infection. J Exp Med 2008;205:2075-84. 163. Bull CG, McKee CM. Respiratory immunity in rabbits. VII. Resistance to intranasal infection in the absence of demonstrable antibodies. Am J Epidemiol 1929;9:490-99. 164. Conley ME, Delacroix DL. Intravascular and mucosal immunoglobulin A: two separate but related systems of immune defense? Ann Intern Med 1987;106:892-99. 165. Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol 2003;3:63-72. 166. Hiller AS, Tschernig T, Kleemann WJ, et al. Bronchus-associated lymphoid tissue (BALT) and larynx-associated lymphoid tissue (LALT) are found at different frequencies in children, adolescents and adults. Scand J Immunol 1998;47:159-162. 167. Tschernig T, Pabst R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology 2000;68:1-8. 168. Gallichan WS, Rosenthal KL. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med 1996;184:1879-90. 81 169. Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005;11:S45-53. 170. Dietrich J, Andersen C, Rappuoli R, et al. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol 2006;177:6353-60. 171. Wang J, Thorson L, Stokes RW, et al. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol 2004;173:6357-65. 172. Santosuosso M, McCormick S, Zhang X, et al. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infect Immun 2006;74:4634-43. 173. Reljic R, Williams A, Ivanyi J. Mucosal immunotherapy of tuberculosis: is there a value in passive IgA? Tuberculosis (Edinb) 2006;86:179-90. 174. Weill-Halle B. in SR Rosenthal (ed,) PSG Publishing Littleton MA 1980;175-181. 175. Oettinger T, Jørgensen M, Ladefoged A, et al. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber Lung Dis 1999;79:243-50. 176. Oettinger T, Andersen ÅB. Cloning and B-cell epitope mapping of MPT64 from Mycobacterium tuberculosis H37Rv. Infect Immun 1994;62:2058–2064. 177. Li H, Ulstrup JC, Jonassen TØ, et al. Evidence for absence of the MPB64 gene in some substrains of Mycobacterium bovis BCG. Infect Immun 1993;61:1730–34. 178. Dubos RJ, Pierce CH. Differential characteristics in vitro and in vivo of several substrains of BCG. IV. Immunizing effectiveness. Am Rev Tuberc 1956;74:699–714. 179. Gheorghiu M, Lagrange PH, Fillastre C. The stability and immunogenicity of a dispersed-grown freeze-dried Pasteur BCG vaccine. J Biol Stand 1988;16:15–26. 180. Milstein JB, Gibson JJ. Quality control of BCG vaccines by the World Health Organization: a review of factors that may influence vaccine effectiveness and safety. WHO/EPI/Gen/89.1, World Health Organization, Geneva, 1989. 181. Kovarik J, Bozzotti P, Love-Homan L, et al. CpG oligodeoxynucleotides can circumvent the Th2 polarization of neonatal responses to vaccines but may fail to fully redirect Th2 responses established by neonatal priming. J Immunol 1999;162:1611-7. 182. Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science 1996;271:1728-30. 183. Barrios C, Brandt C, Berney M, et al. Partial correction of the TH2/TH1 imbalance in neonatal murine responses to vaccine antigens through selective adjuvant effects. Eur J Immunol 1996;26:2666-70. 184. Adkins B, Ghanei A, Hamilton K. Up-regulation of murine neonatal T helper cell responses by accessory cell factors. J Immunol 1994 Oct 15;153(8):3378-85. 185. Jones CA, Holloway JA, Warner JO. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. J Reprod Immunol 2002;56:45-60. 82 186. Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007;7:379-90. 187. Siegrist CA. Neonatal and early life vaccinology. Vaccine 2001 May 14;19(25-26):3331-46. 188. Poole JA, Claman HN. Immunology of pregnancy. Implications for the mother. Clin Rev Allergy Immunol 2004 Jun;26(3):161-70. 189. Dealtry GB, O'Farrell MK, Fernandez N. The Th2 cytokine environment of the placenta. Int Arch Allergy Immunol 2000;123(2):107-19. 190. Sarvas H, Kurikka S, Seppala IJ, et al. Maternal antibodies partly inhibit an active antibody response to routine tetanus toxoid immunization in infants. J Infect Dis 1992;165:977–9. 191. Bjorkholm B, Granstrom M, Taranger J, et al. Influence of high titers of maternal antibody on the serologic response of infants to diphtheria vaccination at three, five and twelve months of age. Pediatr Infect Dis J 1995;14:846–50. 192. Claesson BA, Schneerson R, Robbins JB, et al. Protective levels of serum antibodies stimulated in infants by two injections of Haemophilus influenzae type b capsular polysaccharide–tetanus toxoid conjugate. J Pediatr 1989;114:97–100. 193. Daum RS, Siber GR, Ballanco GA, et al. Serum anticapsular antibody response in the first week after immunization of adults and infants with the Haemophilus influenzae type b-Neisseria meningitidis outer membrane protein complex conjugate vaccine. J Infect Dis 1991;164:1154–9. 194. Kanra G, Yalcin SS, Ceyhan M, et al. Clinical trial to evaluate immunogenicity and safety of inactivated hepatitis A vaccination starting at 2-month-old children. Turk J Pediatr 2000;42:105–8. 195. Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009;9:185-94. 196. Siegrist CA, Cordova M, Brandt C, et al. Determinants of infant responses to vaccines in presence of maternal antibodies. Vaccine 1998;16:1409–14. 197. Siegrist CA, Barrios C, Martinez X, et al. Influence of maternal antibodies on vaccine responses: inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur J Immunol 1998;28:4138–48. 198. Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol 2004;4:553-64. 199. Vekemans J, Amedei A, Ota MO, et al. Neonatal bacillus Calmette-Guérin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol 2001;31:1531-5. 200. Lee CJ. Maternal-foetal interaction, antibody formation, and metabolic response in mice immunized with pneumococcal polysacharides. Immunology 1980;41:45-54. 201. Malhotra I, Ouma J, Wamachi A, et al. In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J Clin Invest 1997;99:1759-66. 202. Elias D, Akuffo H, Pawlowski A, et al. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine 2005;23:1326-34. 83 203. Doherty TM, Rook G. Progress and hindrances in tuberculosis vaccine development. Lancet 2006;367:947-9. 204. Temmerman S, Pethe K, Parra M, et al. Methylation-dependent T cell immunity to Mycobacterium tuberculosis heparin-binding hemagglutinin. Nat Med 2004;10:935-41. 205. Dietrich J, Aagaard C, Leah R, et al. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol 2005;174:6332-9. 206. Brandt L, Elhay M, Rosenkrands I, et al. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun 2000;68:791. 207. Olsen AW, Williams A, Okkels LM, et al. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect Immun 2004;72:6148-50. 208. Agger EM, Rosenkrands I, Olsen AW, et al. Protective immunity to tuberculosis with Ag85BESAT-6 in a synthetic cationic adjuvant system IC31. Vaccine 2006;24:5452-60. 209. Aagaard C, Hoang TT, Izzo A, et al. Protection and polyfunctional T cells induced by Ag85BTB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One 2009;4:e5930. 210. Skeiky YAW, Alderson MR, Guderian JA, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72f, delivered as naked DNA or as recombinant protein. J. Immunol 2004;172:7618-28. 211. Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun 1994;62:2536-44. 212. Roberts AD, Sonnenberg MG, Ordway DJ, et al. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology 1995;85:502-8. 213. Fan X, Gao Q, Fu R. Differential immunogenicity and protective efficacy of DNA vaccines expressing proteins of Mycobacterium tuberculosis in a mouse model. Microbiol Res 2009;164:37482. 214. Doherty TM, Olsen AW, Weischenfeldt J, et al. Comparative analysis of different vaccine constructs expressing defined antigens from Mycobacterium tuberculosis. J Infect Dis 2004;190:214653. 215. Radosevic K, Wieland CW, Rodriguez A, et al. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect Immun. 2007;75:4105-15. 216. Guleria I, Teitelbaum R, McAdam RA, et al. Auxotrophic vaccines for tuberculosis. Nat Med 1996;2:334-7. 217. Horwitz MA, Harth G, Dillon BJ, et al. Enhancing the protective efficacy of Mycobacterium bovis BCG vaccination against tuberculosis by boosting with the Mycobacterium tuberculosis major secretory protein. Infect Immun 2005;73:4676-83. 84 218. Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun 2003;71:1672-9. 219. Xu Y, Zhu B, Wang Q, et al. Recombinant BCG coexpressing Ag85B, ESAT-6 and mouse-IFNgamma confers effective protection against Mycobacterium tuberculosis in C57BL/6 mice. FEMS Immunol Med Microbiol 2007;51:480-7. 220. Grode L, Seiler P, Baumann S, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J Clin Invest 2005;115:2472-9. 221. Williams A, Hatch GJ, Clark SO, et al. Evaluation of vaccines in the EU TB Vaccine Cluster using a guinea pig aerosol infection model of tuberculosis. Tuberculosis (Edinb) 2005 ;85:29-38. 222. Brooks JV, Frank AA, Keen MA, et al. Boosting vaccine for tuberculosis. Infect Immun 2001;69:2714-7. 223. Skeiky YA, Dietrich J, Lasco TM, et al. Non-clinical efficacy and safety of HyVac4:IC31 vaccine administered in a BCG prime-boost regimen. Vaccine. Vaccine 2010 ;28:1084-93. 224. Fan X, Gao Q, Fu R. DNA vaccine encoding ESAT-6 enhances the protective efficacy of BCG against Mycobacterium tuberculosis infection in mice. Scand J Immunol 2007 ;66:523-8. 225. Dietrich J, Billeskov R, Doherty TM, et al. Synergistic effect of bacillus calmette guerin and a tuberculosis subunit vaccine in cationic liposomes: increased immunogenicity and protection. J Immunol 2007;178:3721-30. 226. Clark TW, Pareek M, Hoschler K, et al. Trial of Influenza A (H1N1) 2009 Monovalent MF59Adjuvanted Vaccine -- Preliminary Report. N Engl J Med 2009;361:2424-35. 227. van Dissel JT, Arend SM, Prins C, et al. Ag85B-ESAT-6 adjuvanted with IC31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naïve human volunteers. Vaccine 2010;28:3571-81. 228. Cole ST, Brosch R, Parkhill J, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998;393:537-44. 229. Masungi C, Temmerman S, Van Vooren JP, et al. Differential T and B cell responses against Mycobacterium tuberculosis heparin-binding hemagglutinin adhesin in infected healthy individuals and patients with tuberculosis. J Infect Dis 2002;185:513-520. 230. Prieur E, Gilbert SC, Schneider J, et al. A Plasmodium falciparum candidate vaccine based on a six-antigen polyprotein encoded by recombinant poxviruses. Proc Natl Acad Sci U S A 2004;101:2905. 231. Coler RN, Skeiky YA, Bernards K, et al. Immunization with a polyprotein vaccine consisting of the T-Cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infect Immun 2002;70:4215-25. 232. Vajdy M, Selby M, Medina-Selby A, et al. Hepatitis C virus polyprotein vaccine formulations capable of inducing broad antibody and cellular immune responses. J Gen Virol 2006;87:2253-62. 85 233. Skeiky YA, Alderson MR, Ovendale PJ, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol 2004;172:7618-28. 234. Silva CL, Palacios A, Colston MJ et al. Mycobacterium leprae 65hsp antigen expressed from a retroviral vector in a macrophage cell line is presented to T cells in association with MHC class II in addition to MHC class I. Microbial Pathogenesis 1992;12:27-38. 235. Silva CL, Lowrie DB. A single mycobacterial protein (hsp 65) expressed by a transgenic antigenpresenting cell vaccinates mice against tuberculosis. Immunology 1994;82:244-8. 236. Huygen K, Content J, Denis O, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med 1996;2:893-8. 237. Khera A, Singh R, Shakila H, et al. Elicitation of efficient protective immune responses by using DNA vaccines against tuberculosis. Vaccine 2005;23:5655-65. 238. Baldwin SL, D'Souza C, Roberts AD, et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun 1998;66:2951-9. 239. Orme IM, McMurray DN, Belisle JT. Tuberculosis vaccine development: recent progress. Trends Microbiol 2001;9(3):115-8. 240. Smith DA, Parish T, Stoker NG, et al. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect Immun 2001 ;69:114250. 241. Pym AS, Brodin P, Majlessi L, et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 2003;9:533-9. 242. Bao L, Chen W, Zhang H, et al. Virulence, immunogenicity, and protective efficacy of two recombinant Mycobacterium bovis bacillus Calmette-Guérin strains expressing the antigen ESAT-6 from Mycobacterium tuberculosis. Infect Immun 2003;71:1656-61. 243. Hoft DF, Blazevic A, Abate G, et al. A new recombinant bacille Calmette-Guérin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis 2008;198:1491-501. 244. Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol 2006;4:469-76. 245. McShane H, Pathan AA, Sander CR, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med 2004;10:1240-4. 246. McShane H, Pathan AA, Sander CR, et al. Boosting BCG with MVA85A: the first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis (Edinb) 2005;85:47-52. 247. Scriba TJ, Tameris M, Mansoor N, et al. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol 2010;40:279-90. 248. Rouanet C, Debrie AS, Lecher S, et al. Subcutaneous boosting with heparin binding haemagglutinin increases BCG-induced protection against tuberculosis. Microbes Infect 2009 ;11:9951001. 86 249. Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708-12. 250. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004;22:745-63. 251. Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006;107:4781-9. 252. De Rosa SC, Lu FX, Yu J, et al. Vaccination in humans generates broad T cell cytokine responses. J Immunol 2004;173:5372-80. 253. Betts MR, Exley B, Price DA, et al. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced T cell responses and their role in protection after HIV-1 infection. Proc Natl Acad Sci U S A 2005;102:4512-7. 254. Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med 2007;204:1405-16. 255. Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccinemediated protection against Leishmania major. Nat Med 2007;13:843-50. 256. Palma C, Iona E, Giannoni F, et al. The Ag85B protein of Mycobacterium tuberculosis may turn a protective immune response induced by Ag85B-DNA vaccine into a potent but non-protective Th1 immune response in mice. Cell Microbiol 2007;9:1455-65. 257. Beveridge NE, Price DA, Casazza JP, et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol 2007;37:3089-100. 258. Tchilian EZ, Desel C, Forbes EK, et al. Immunogenicity and protective efficacy of prime-boost regimens with recombinant (delta)ureC hly+ Mycobacterium bovis BCG and modified vaccinia virus ankara expressing M. tuberculosis antigen 85A against murine tuberculosis. Infect Immun 2009;77:622-31. 259. Forbes EK, Sander C, Ronan EO, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 2008;181:4955-64. 260. Holmgren J, Harandi AM, Czerkinsky C. Mucosal adjuvants and anti-infection and antiimmunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Expert Rev. Vaccines 2003;2:205−217. 261. Plant A, Williams NA. Modulation of the immune response by the cholera-like enterotoxins. Curr Top Med Chem 2004;4:509−519. 262. Harandi AM, Holmgren J. CpG DNA as a potent inducer of mucosal immunity: implications for immunoprophylaxis and immunotherapy of mucosal infections. Curr Opin Investig Drugs 2004;5:141−145. 263. Chen L, Wang J, Zganiacz A, et al. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect Immun 2004;72:238-46. 87 264. Rodríguez A, Troye-Blomberg M, Lindroth K, et al. B- and T-cell responses to the mycobacterium surface antigen PstS-1 in the respiratory tract and adjacent tissues. Role of adjuvants and routes of immunization. Vaccine 2003;21:458-67. 265. Santosuosso M, Zhang X, McCormick S, et al. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol 2005;174:7986-94. 266. Smithwick RW. Laboratory manual for acid-fast microscopy (2nd edition). Center for Disease Control, Atlanta, 1975. 267. Peterson EM, Nakasone A, Platon-DeLeon JM, et al. Comparison of direct and concentrated acid-fast smears to identify specimens culture positive for Mycobacterium spp. Clin Microbiol 1999;37:3564-8. 268. Arend SM, van Soolingen D, Ottenhoff TH, et al. Repeatedly negative tuberculin skin tests followed by active tuberculosis in an immunocompetent individual. Neth J Med. 2001 ;58:76-81. 269. Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med 2006;355:1539-50. 270. Palomino JC. Nonconventional and new methods in the diagnosis of tuberculosis: feasibility and applicability in the field. Eur Respir J 2005;26:339-50. 271. Steingart KR, Dendukuri N, Henry M, et al. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol 2009 ;16:260-76. 272. Julián E, Matas L, Pérez A, et al. Serodiagnosis of tuberculosis: comparison of immunoglobulin A (IgA) response to sulfolipid I with IgG and IgM responses to 2,3-diacyltrehalose, 2,3,6triacyltrehalose, and cord factor antigens. J Clin Microbiol 2002 ;40:3782-8. 273. Gey van Pittius NC, Warren RM, van Helden PD. ESAT-6 and CFP-10: what is the diagnosis? Infect Immun 2002;70:6509-10. 274. Zhang H, Wang J, Lei J, et al. PPE protein (Rv3425) from DNA segment RD11 of Mycobacterium tuberculosis: a potential B-cell antigen used for serological diagnosis to distinguish vaccinated controls from tuberculosis patients. Clin Microbiol Infect 2007 ;13:139-45. 275. El-Masry S, El-Kady I, Zaghloul MH, et al. Rapid and simple detection of a mycobacterium circulating antigen in serum of pulmonary tuberculosis patients by using a monoclonal antibody and Fast-Dot-ELISA. Clin Biochem 2008;41:145-51. 276. Kang YA, Lee HW, Yoon HI, et al. Discrepancy between the tuberculin skin test and the wholeblood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA 2005;293:2756-61. 277. Farris A, Branda J. QuantiFERON-TB gold assay for tuberculosis infection. Clinical Microbiology Newsletter 2007;29:129-136 278. Rodriguez A, Tjarnlund A, Ivanji J, et al. Role of IgA in the defense against respiratory infections: IgA deficient mice exhibited increased susceptibility to intranasal infection with Mycobacterium bovis BCG. Vaccine 2005; 23: 2565-2572. 88 279. Araujo Z, Waard JH, Fernández de Larrea C, et al. Study of the antibody response against Mycobacterium tuberculosis antigens in Warao Amerindian children in Venezuela. Mem Inst Oswaldo Cruz 2004;99:517-24. 280. Hamasur B, Bruchfeld J, Haile M, et al. Rapid diagnosis of tuberculosis by detection of mycobacterial lipoarabinomannan in urine. J Microbiol Methods 2001;45:41-52. 281. Whittaker E, Gordon A, Kampmann B. Is IP-10 a better biomarker for active and latent tuberculosis in children than IFNgamma? PLoS One. 2008;3:e3901. 282. Lit LC, Wong CK, Tam LS, et al. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann Rheum Dis 2006;65:209–215. 283. Romagnani P, Rotondi M, Lazzeri E, et al. Expression of IP-10/CXCL10 and MIG/CXCL9 in the thyroid and increased levels of IP-10/CXCL10 in the serum of patients with recent-onset Graves' disease. Am J Pathol 2002;161:195–206. 284. Kawamura A, Miura S, Fujino M, et al. CXCR3 chemokine receptor-plasma IP10 interaction in patients with coronary artery disease. Circ J 2003;67:851–854. 285. Jinquan T, Jing C, Jacobi HH, et al. CXCR3 expression and activation of eosinophils: role of IFN-gamma-inducible protein-10 and monokine induced by IFN-gamma. J Immunol 2000;165:1548– 56. 286. Phillips M, Cataneo RN, Condos R, et al. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis (Edinb) 2007;87:44-52. 287. Küpeli E, Karnak D, Beder S, et al. Diagnostic accuracy of cytokine levels (TNF-alpha IL-2 and IFN-gamma) in bronchoalveolar lavage fluid of smear-negative pulmonary tuberculosis patients. Respiration 2008;75:73-8. 288. Jacobsen M, Repsilber D, Gutschmidt A, et al. Candidate biomarkers for discrimination between infection and disease caused by Mycobacterium tuberculosis. J Mol Med 2007;85:613-21. 289. Jónsdóttir I. Maturation of mucosal immune responses and influence of maternal antibodies. J Comp Pathol 2007;137:S20-6. 290. Kaufmann S. Protection against tuberculosis: cytokines, T cells, and macrophages. Ann Rheum Dis 2002; 61: ii54–ii58. 291. Daugelat S, Ladel CH, Kaufmann SH. Influence of mouse strain and vaccine viability on T-cell responses induced by Mycobacterium bovis bacillus Calmette-Guérin. Infect Immun 1995; 63:203340. 292. Taha RA, Kotsimbos TC, Song YL, et al. IFN-gamma and IL-12 are increased in active compared to inactive tuberculosis. Am J Respir Crit Care Med 1997;55:1135-39. 293. Condos R, Rom WN, Liu YM et al. Local immune responses correlated with presentation and outcome in tuberculosis. Am J Respir Crit Care Med 1998;157:729-35. 294. Lawn SD, Rudolph D, Wiktor S, et al. Tuberculosis (TB) and HIV infection are independently associated with elevated serum concentrations of tumour necrosis factor receptor type 1 and beta2microglobulin respectively. Clin Exp Immunol 2000;122:79-84. 89 295. Ferebee SH, Mount FW, Murray FJ, et al. A controlled trial of isoniazid prophylaxis in mental institutions. Am Rev Respir Dis. 1963;88:161-75. 296. Opie EL, Aronson DJD. Tubercle bacilli in latent tuberculous lesions and in lung tissue without tuberculous lesions. Arch Path Lab 1927;4:1. 297. Robertson HE. Persistence of tuberculous infection. Am J Pathol 1933;9:711. 298. Liu KD, Glidden DV, Eisner MD, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med 2007;35:2755-61. 299. Mattey DL, Glossop JR, Nixon NB et al. Circulating levels of tumor necrosis factor receptors are highly predictive of mortality in patients with rheumatoid arthritis. Arthritis Rheum 2007;56:3940-8. 300. Kakumu S, Okumura A, Ishikawa T, et al. Serum levels of IL-10 IL-15 and soluble tumour necrosis factor-alpha (TNF-alpha) receptors in type C chronic liver disease. Clin Exp Immunol 1997;109:458-63. 301. Xing Z, Charters TJ. Heterologous boost vaccines for bacillus Calmette-Guérin prime immunization against tuberculosis. Expert Rev Vaccines 2007;6:539-46. 302. Reed SG, Coler RN, Dalemans W, et al. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci U S A 2009;106:2301-6. 303. Elvang T, Christensen JP, Billeskov R, et al. CD4 and CD8 T cell responses to the M. tuberculosis Ag85B-TB10.4 promoted by adjuvanted subunit, adenovector or heterologous prime boost vaccination. PLoS One 2009;4:e5139. 304. Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol 2009;21:346-51. 305. Vekemans J, Amedei A, Ota MO, et al. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol 2001;31:1531-5. 306. Buddle BM, Wedlock DN, Parlane NA, et al. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect Immun 2003;71:6411-9. 307. Corner LA, Buddle BM, Pfeiffer DU, et al. Vaccination of the brushtail possum (Trichosurus vulpecula) against Mycobacterium bovis infection with bacille Calmette-Guérin: the response to multiple doses. Vet Microbiol. 2002;84:327-36. 308. Rodrigues LC, Pereira SM, Cunha SS, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC cluster-randomised trial. Lancet 2005;366:1290-5. 309. Dourado I, Rios MH, Pereira SM, et al. Rates of adverse reactions to first and second doses of BCG vaccination: results of a large community trial in Brazilian schoolchildren. Int J Tuberc Lung Dis 2003;7:399-402. 310. Mazzantini RP, Miyaji EN, Dias WO, et al. Adjuvant activity of Mycobacterium bovis BCG expressing CRM197 on the immune response induced by BCG expressing tetanus toxin fragment C. Vaccine 2004;22:740-6. 311. Matsumoto S, Yukitake H, Kanbara H, et al. Recombinant Mycobacterium bovis bacillus Calmette-Guérin secreting merozoite surface protein 1 (MSP1) induces protection against rodent 90 malaria parasite infection depending on MSP1-stimulated interferon gamma and parasite-specific antibodies. J Exp Med 1998;188:845-54. 312. Badell E, Nicolle F, Clark S, et al. Protection against tuberculosis induced by oral prime with Mycobacterium bovis BCG and intranasal subunit boost based on the vaccine candidate Ag85BESAT-6 does not correlate with circulating IFN-gamma producing T-cells. Vaccine 2009;27:28-37. 313. Brandt L, Skeiky YA, Alderson MR, et al. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect Immun 2004;72:6622-32. 314. Kolibab K, Parra M, Yang AL, et al. A practical in vitro growth inhibition assay for the evaluation of TB vaccines. Vaccine 2009;28:317-22. 315. Parra M, Yang AL, Lim J, et al. Development of a murine mycobacterial growth inhibition assay for evaluating vaccines against Mycobacterium tuberculosis. Clin Vaccine Immunol 2009;16:1025-32. 316. Giri PK, Schorey JS. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One 2008;3:e2461. 317. Hovav AH, Mullerad J, Davidovitch L, et al. The Mycobacterium tuberculosis recombinant 27kilodalton lipoprotein induces a strong Th1-type immune response deleterious to protection. Infect Immun 2003;71:3146-54. 318. Kohama H, Umemura M, Okamoto Y, et al. Mucosal immunization with recombinant heparinbinding haemagglutinin adhesin suppresses extrapulmonary dissemination of Mycobacterium bovis bacillus Calmette-Guérin (BCG) in infected mice. Vaccine 2008;26:924-32. 319. Giri PK, Sable SB, Verma I, et al. Comparative evaluation of intranasal and subcutaneous route of immunization for development of mucosal vaccine against experimental tuberculosis. FEMS Immunol Med Microbiol 2005;45:87-93. 320. Carpenter ZK, Williamson ED, Eyles JE. Mucosal delivery of microparticle encapsulated ESAT6 induces robust cell-mediated responses in the lung milieu. J Control Release 2005 5;104:67-77. 321. Xing Z, McFarland CT, Sallenave JM, et al. Intranasal mucosal boosting with an adenovirusvectored vaccine markedly enhances the protection of BCG-primed guinea pigs against pulmonary tuberculosis. PLoS One 2009;4:e5856. 322. Ota MO, Vekemans J, Schlegel-Haueter SE, et al. Influence of Mycobacterium bovis bacillus Calmette-Guérin on antibody and cytokine responses to human neonatal vaccination. J Immunol 2002;168: 919-25. 323. Chambers MA, Marshall BG, Wangoo A, et al. Differential responses to challenge with live and dead Mycobacterium bovis Bacillus Calmette-Guérin. J Immunol 1997;158:1742-8. 324. Demkow T, Alter A, Wiechno P. Intravesical bacillus Calmette-Guérin therapy for T1 superficial bladder cancer. Urol Int 2008;80:74-9. 325. Herz U, Joachim R, Ahrens B, et al. Allergic Sensitization and Allergen Exposure during Pregnancy Favor the Development of Atopy in the Neonate. Int Arch Allergy Immunol 2001; 124:193–6. 326. Devereux G, Seaton A, Barker RN. In utero priming of allergen-specific helper T cells. Clin Exp Allergy 2001;31:1686–95. 91 327. Carlier Y, Nzeyimana H, Bout D, et al. Evaluation of circulating antigens by a sandwich radioimmunoassay, and of antibodies and immune complexes, in Schistosoma mansoni-infected African parturients and their newborn children. Am J Trop Med Hyg 1980;29:74–81. 328. Pravieux JJ, Poulet H, Charreyre C, et al. Protection of newborn animals through maternal immunization. J Comp Pathol 2007;137: S32-4. 329. Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008;359:1555-64. 330. Szépfalusi Z, Loibichler C, Pichler J, et al. Direct evidence for transplacental allergen transfer. Pediatr Res 2000;48:404-7. 331. Holsapple MP, West LJ, Landreth KS. Species comparison of anatomical and functional immune system development. Birth Defects Res B Dev Reprod Toxicol. 2003;68:321–34. 332. Marchant A, Appay V, Van Der Sande M, et al. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest 2003;111:1747–55. 333. Talbot EA, Perkins MD, Silva SF, et al. Disseminated bacille Calmette-Guérin disease after vaccination: case report and review. Clin Infect Dis 1997;24:1139-46. 334. Underhill DM, Ozinsky A, Smith KD, et al. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A 1999;96:14459-63. 335. Tjärnlund A, Guirado E, Julián E, et al. Determinant role for Toll-like receptor signalling in acute mycobacterial infection in the respiratory tract. Microbes Infect 2006;8:1790-800. 336. Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 1999;285:732-36. 337. Harris D, Vordermeier H, Roman E, et al. Murine T cell stimulatory peptides from the 19-kDa antigen of Mycobacterium tuberculosis: epitope restricted homology with the 28kDa protein of Mycobacterium leprae. J Immunol 1991;147:2706–12. 338. Rao V, Dhar N, Shakila H, et al. Increased expression of Mycobacterium tuberculosis 19 kDa lipoprotein obliterates the protective efficacy of BCG by polarizing host immune responses to the Th2 subtype. Scand J Immunol 2005;61:410-7. 339. Diaz-Silvestre H, Espinosa-Cueto P, Sanchez-Gonzalez A, et al. The 19-kDa antigen of Mycobacterium tuberculosis is a major adhesin that binds the mannose receptor of THP-1 monocytic cells and promotes phagocytosis of mycobacteria. Microb Pathog 2005;39:97-107. 340. Sallusto F, Cella M, Danieli C, et al. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med 1995 ;182:389-400. 341. Noss EH, Pai RK, Sellati TJ, et al. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol 2001;167:910-18. 342. Fulton SA, Reba SM, Pai RK, et al. Inhibition of major histocompatibility complex II expression and antigen processing in murine alveolar macrophages by Mycobacterium bovis BCG and the 19kilodalton mycobacterial lipoprotein. Infect Immun 2004;72:2101-10. 92 343. Arko-Mensah J, Julián E, Singh M, et al. TLR2 but not TLR4 signalling is critically involved in the inhibition of IFN-gamma-induced killing of mycobacteria by murine macrophages. Scand J Immunol 2007;65:148-57. 344. Rao V, Dhar N, Tyagi AK. Modulation of host immune responses by overexpression of immunodominant antigens of Mycobacterium tuberculosis in bacille Calmette-Guérin. Scand J Immunol 2003;58:449-61. 93