* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download FROM PATHWAYS TO PEOPLE: ALLERGIC CONTACT DERMATITIS GAVIN MAXWELL
DNA vaccination wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Adaptive immune system wikipedia , lookup
Innate immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Molecular mimicry wikipedia , lookup
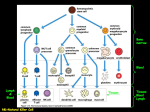
FROM PATHWAYS TO PEOPLE: MODELLING ALLERGIC CONTACT DERMATITIS GAVIN MAXWELL SAFETY & ENVIRONMENTAL ASSURANCE CENTRE (SEAC) UNILEVER R&D ALLERGIC CONTACT DERMATITIS 1. Skin penetration and haptenation: covalent modification of skin protein 9. Recruitment of antigenspecific memory T cells and expansion of effector T cells to elicit response 2. Migration of Langerhans cells and dermal dendritic cells 8. Processing and presentation by skin APCs 3. Antigen processing and presentation by dendritic cells 4. Presentation of haptenated peptide by dendritic cell to T cells 5. Proliferation and differentiation of specific T cells 6. Generation of antigen-specific memory T cell population Image from: Karlberg et al. Chem. Res. Toxicol. (2008), 21, 53-69. 7. Re-exposure to chemical CURRENT HUMAN HEALTH RISK ASSESSMENT PARADIGM FOR CHEMICAL INGREDIENTS NOAEL No Observed Adverse Effect Level (NOAEL) ÷ 10 - 1000 NEW HUMAN HEALTH RISK ASSESSMENT PARADIGM FOR SENSITISING INGREDIENTS? Allergic Immune Response dose Y Adverse Non-Adverse dose X Time NEW HUMAN HEALTH RISK ASSESSMENT PARADIGM FOR SENSITISING INGREDIENTS? 1. Skin Penetration 2. Electrophilic substance: directly or via auto-oxidation or metabolism 3-4. Haptenation: covalent modification of epidermal proteins 5-6. Activation of epidermal keratinocytes & Dendritic cells 7. Presentation of haptenated protein by Dendritic cell resulting in activation & proliferation of specific T cells 8-11. Allergic Contact Dermatitis: Epidermal inflammation following re-exposure to substance due to T cell-mediated cell death Modified from ‘Adverse Outcome Pathway (AOP) for Skin Sensitisation’, OECD DEVELOP A MATHEMATICAL MODEL OF ALLERGIC CONTACT DERMATITIS TO ENABLE RISK ASSESSMENT DECISION-MAKING FOR NEW CHEMICALS Vehicle Lymphatics Draining Lymph Nodes Blood/resting lymphatics Epidermis LC Dermis dDC LC dDC Key Chemical Protein Dendritic cell CD8+ T cell: N = naïve; CM = central memory; EM = effector memory; E = effector DC N N DC CM CM PM EM EM E E E Skin KEY ASSUMPTION: ANTIGEN DRIVING T CELL RESPONSE IS HAPTENATED PEPTIDE Direct Acting - haptenated residues present on pMHC initiating the response &/or Altered Processing - haptenated residues disrupt normal proteaosome processing resulting in presentation of altered self-peptides &/or Altered Selection - hapten activity disrupts MHC loading resulting in altered selection of self Dendritic cell T cell PREDICTING HAPTENATION RATE OF SKIN PROTEIN BY DI-NITROCHLOROBENZENE (DNCB) • Modelling approach - treat proteins as mixture of nucleophilic residues • Use experimental data to determine ‘bulk’ haptenation rate & estimate the fraction of nucleophiles we expect to be haptenated Solution F Concentration 210 205 200 195 190 185 180 175 170 0 2 4 6 8 10 12 14 16 18 20 22 24 26 Cys Tyr Lys His Arg Met & N-term 300 250 Concentration 215 200 150 100 50 0 0 2 4 6 8 10 Time (hours) T Bound in Skin 150 100 Key Chemical Protein Nucleophilic residue Concentration Skin Concentration 80 100 50 0 0 2 4 6 8 10 12 14 16 18 Time (hours) 20 22 24 26 60 40 20 0 0 2 4 6 8 10 T MODELLING SKIN BIOAVAILABILITY OF CHEMICAL Donor chamber Skin position Receptor chamber Loss from formulation Window Receptor solution out Partitioning Diffusion Loss from skin Davies et al. 2011. Toxicol Sci. 119. 308-18 PREDICTING EXTENT OF SKIN PROTEIN HAPTENATION FOLLOWING SINGLE EXPOSURE TO DNCB Skin bioavailability model expanded to include covalent modification of skin protein by chemical » Amount of haptenated protein predicted over time Solution Free in Skin 215 300 210 Concentration Concentration 250 205 200 195 190 185 180 200 150 100 50 175 170 0 2 4 6 8 10 12 14 16 18 20 22 24 0 26 0 2 4 6 8 10 Time (hours) 12 14 16 18 20 22 24 26 20 22 24 26 Time (hours) Bound in Skin Protein 150 100 Concentration Concentration 80 100 50 0 0 2 4 6 8 10 12 14 16 18 Time (hours) 20 22 24 26 60 40 20 0 0 2 4 6 8 10 12 14 16 18 Time (hours) » Haptenated protein and free chemical concentrations are inputs to immune response model Davies et al. 2011. Toxicol Sci. 119. 308-18 TRANSLATING CHEMICAL SENSITISER EXPOSURE INTO EXTENT OF HAPTEN PRESENTATION Formulation Lymphatics Epidermis LC Dermis dDC Dendritic cell Draining Lymph Nodes Blood/resting lymphatics LC dDC DC N N DC CM CM PM EM EM E E E Skin T cell • Intracellular LC/DC protein is haptenated by free chemical • Proteasomal processing and Class I MHC presentation • DC-T cell synapse in draining lymph node MODELLING PROTEASOMAL PROCESSING & CLASS I MHC ANTIGEN PRESENTATION Assume ‘Direct Acting’ hypothesis (unaltered proteasomal processing) and determine properties of resulting peptides Prediction tools Proteasomal cleavage (e.g. NetChop) average number of pMHC generated per protein MHC I binding (e.g. NetMHCpan) average number of nucleophiles per pMHC Estimate average pMHC surface density from considerations of: 1. the fraction of nucleophiles we expect to be haptenated 2. probability that a pMHC contains a haptenated nucleophile ILLUSTRATION FROM YEWDELL, J.W., E. REITS, AND J. NEEFJES. (2003). Making sense of mass destruction: quantitating MHC class I antigen presentation. Nat. Rev. Immunol. 3, 952–61. VITA, R., L. ZAREBSKI, J.A. GREENBAUM, ET AL. (2010). The immune epitope database 2.0. Nucleic Acids Res. 38, D854–62. MODELLING DC-T CELL INTERACTIONS IN DRAINING LYMPH NODE Formulation Lymphatics Epidermis LC Dermis dDC Draining Lymph Nodes Blood/resting lymphatics LC dDC DC N N DC CM CM PM EM EM E E E Skin • LC/dDC migrate from sensitiser-exposed skin to present haptenated peptides via Class I MHC to CD8+ T cell in draining lymph node e.g. Pickard et al, 2009 • DC/T cell movement in lymph node is described by random walk e.g. Day & Lythe, 2012 DEVELOP A MATHEMATICAL MODEL OF ALLERGIC CONTACT DERMATITIS TO ENABLE RISK ASSESSMENT DECISION-MAKING FOR NEW CHEMICALS Solution Free in Skin 215 300 210 Concentration Concentration 250 205 200 195 190 185 180 200 150 100 50 175 170 0 2 4 6 8 10 12 14 16 18 20 22 24 0 26 0 2 4 6 8 10 Time (hours) Haptenated protein Bound in Skin Vehicle 12 14 16 18 20 22 24 26 Time (hours) Protein 150 100 LC 50 0 0 2 4 6 8 10 12 14 16 18 Time (hours) Dermis dDC 20 22 24 26 Allergic Immune ResponseConcentration Epidermis Concentration 80 100 60 dose Y 40 20 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 Time (hours) Adverse Non-Adverse dose X Time ‘T LYMPHOCYTES: ORCHESTRATORS OF SKIN SENSITISATION’ WORKSHOP – MAY 2010, LONDON Immunologists, risk assessors & mathematical modellers – 2 day workshop What are the characteristics of the T cell response that could reflect sensitiser potency in humans? Number of T lymphocytes » Magnitude: What is the extent of sensitiser-induced T cell response (volume, kinetics & duration)? » Quality: Within sensitiser-induced T cell response, what is the balance between the T cell sub-populations? » Breadth: What proportion of the T cell clonal repertoire has been stimulated by a given sensitiser? Weaker allergen Stronger allergen Treg CD8+ CD8+ Treg Kimber et al. 2012. Toxicology. 291. 18-24 Time CD8+ T CELL RESPONSE: INITIAL MODEL SCOPE Current model scope models the antigen-specific CD8+ T cell response including: » naïve (N) - CD45RO-veCD62L+ve or CD45RA+veCD27+ve » central memory (CM) CD45RO+veCD62L+ve or CD45RA-veCD27+ve » effector memory (EM) CD45RO+veCD62L-ve or CD45RA-veCD27-ve » effector (E) - CD45RO-veCD62Lve or CD45RA+veCD27-ve Human sensitiser-specific T cell data is largely unavailable: » Make use of literature data » Generate sensitiser-specific, human-relevant data Draining Lymph Nodes Blood/resting lymphatics Key Chemical Protein Dendritic cell CD8+ T cell: N = naïve; CM = central memory; EM = effector memory; E = effector DC N N DC CM CM PM EM EM E E E Skin PREDICTING THRESHOLD FOR T CELL ACTIVATION Is the nature (TCR affinity) of the antigen limiting? - what kon/koff do TCRs have for cognate hapten pMHC Explore effect of pMHC surface density and kon/koff on probability of T-cell triggering with the available models (Zarnitsyna & Zhu, 2012). Simulations generated using ‘confinement time’ model of Dushek, et al, 2009. Figures from: Huppa & Davis, 2013; Aleksic et al., 2010 PREDICTING THRESHOLD FOR T CELL ACTIVATION Is the nature (TCR affinity) of the antigen limiting? - what kon/koff do TCRs have for cognate hapten pMHC Explore effect of pMHC surface density and kon/koff on probability of T-cell triggering with the available models (Zarnitsyna & Zhu, 2012). Simulations generated using ‘confinement time’ model of Dushek, et al, 2009. Molecular basis of T cell recognition: how do TcRs interact with sensitising antigens? TcR V-J region usage • Thermodynamic and kinetic parameters • Role of MHC • Characteristics of the CDR3s, and framework • MHC Using DeCombinatoR: (//github.com/uclinfectioni mmunity/Decombinator) to assign TcR sequences - V region usage, J region usage, no. of V deletions, no. of J deletions, CDR3 sequence read Benny Chain & Theres Matjeka Number of nucleotides added between V and J segment Number of V germline deletions Number of J germline deletions CD8+ T CELL DIFFERENTIATION: COMPARING CURRENT HYPOTHESES • Experiments tracking T cell fates have generated a range of hypotheses on T cell differentiation • Need to select a differentiation mechanism despite uncertainty to predict the number of CD8+ memory T cells following sensitizer exposure • Currently building CD8+ T cell models based upon both decreasingpotential (Leeds) & asymmetricdivision (Unilever) to explore the impact of each mechanism on predicted T cell response Image from: Kaech and Cui, Nat. Rev. Immunol. (2012), 12, 749-761 Sheeja Krishnan, Grant Lythe & Carmen Molina-Paris STARTING T CELL POPULATION SIZE » Assume no antigen specific effector or memory CD8+ T cells at the start in an unexposed individual » Estimate number of naïve antigen specific CD8+ T cells in DLN & blood » Assume exposure to skin on the arm » 25 draining lymph nodes (DLN) in axilla out of 650 in total » Consider a single TCR » One in 25 million naïve T cells are antigen specific Whole of body 72.5 bn 2900 All LNs All TCRs Ag specific (1 TCR) 19 bn 760 DLN Blood 0.73 bn 29 1.45 bn 58 Vrisekoop et al, 2008, PNAS 105 (16) 6115-6120; Westermann & Pabst, 1992, Clin. Investig. 70 539-544; Arstila et al, 1999, Science 286 958 MODELLING PROGRAMMED T CELL PROLIFERATION CD69 • Following activation, CD8+ T cell proliferation continues independently of further antigenic stimulation • Going through 7-19 generations (Kaech & Ahmed, 2001; Badovinac et al, 2007) to develop effector and memory populations • No human data available for proliferation rates • Obtain proliferation rates from mouse models (e.g. Yoon et al, 2010: draining lymph node response to influenza virus infection) CFSE Figure from: Yoon et al, 2010, PLOS One 5 (11) e15423 Draining Lymph Nodes DC DC N Blood/resting lymphatics N Key Chemical Protein Dendritic cell CD8+ T cell: N = naïve; CM = central memory; EM = effector memory; E = effector CM CM PM EM EM E E E No. T cells CD8+ T CELL MODEL PREDICTIONS: 5 DAY ANTIGEN EXPOSURE IN LYMPH NODE, 1X MODEL ITERATION Time in days Skin • Combine the parameters and processes together • Simulate single exposure to chemical and track response for one month Draining Lymph Nodes DC DC N Blood/resting lymphatics N Key Chemical Protein Dendritic cell CD8+ T cell: N = naïve; CM = central memory; EM = effector memory; E = effector CM CM PM EM EM E E E Frequency of prediction CD8+ T CELL MODEL PREDICTIONS: 5 DAY ANTIGEN EXPOSURE IN LYMPH NODE, 1000X MODEL ITERATIONS No. T cells Skin • Combine the parameters and processes together • Simulate single exposure to chemical and track response for one month Characterising human T lymphocyte responses to chemical allergen p-phenylenediamine (PPD) 0µg/ml PPD 0.01 0.1 Ki-67 CD8 CD4 Allergen driven proliferation of total lymphocytes and individual T cell subsets measured by intracellular Ki-67 expression. Rebecca Dearman, Amy Popple, Ian Kimber & Jason Williams DEVELOP A MATHEMATICAL MODEL OF ALLERGIC CONTACT DERMATITIS TO ENABLE RISK ASSESSMENT DECISION-MAKING FOR NEW CHEMICALS Vehicle Lymphatics Draining Lymph Nodes Blood/resting lymphatics Epidermis LC Dermis dDC LC dDC Key Chemical Protein Dendritic cell CD8+ T cell: N = naïve; CM = central memory; EM = effector memory; E = effector DC N N DC CM CM PM EM EM E E E Skin PATHWAYS-BASED RISK ASSESSMENT FOR SKIN SENSITISATION: APPLICATION OF MATHEMATICAL MODELLING 1. Skin Penetration 2.Electrophilic substance: directly or via auto-oxidation or metabolism 3-4. Haptenation: covalent modification of epidermal proteins 5-6. Activation of epidermal keratinocytes & Dendritic cells 7. Presentation of haptenated protein by Dendritic cell resulting in activation & proliferation of specific T cells 8-11. Allergic Contact Dermatitis: Epidermal inflammation following re-exposure to substance due to T cell-mediated cell death allergic immune response No. CD8+ T cells dose Y Adverse Non-Adverse dose X time 1. Generate skin bioavailability & haptenation data as model input parameters 2. Use linked mathematical models to predict human allergic immune response 3. Apply human immune response model prediction for risk assessment decision 4. If exposure predicted to be non-adverse, verify prediction using clinical data NEXT STEPS: CHALLENGES AHEAD • Broadening current model scope to include: - CD4+ T helper & regulatory T cell responses - sensitiser-induced inflammation in skin – induction & elicitation - impact of varying frequency & surface area of sensitiser exposure - impact of varying formulation (vehicle) • Using experimental & clinical data to inform & benchmark initial model predictions ACKNOWLEDGEMENTS Unilever Richard Cubberley, Seraya Dhadra, Michael Davies, Nikki Gellatly, Stephen Glavin, Todd Gouin, Sandrine Jacquoilleot, Cameron MacKay, Craig Moore, Ruth Pendlington, Juliette Pickles, Ouarda Saib, David Sheffield, Richard Stark, Vicki Summerfield & Sam Windebank University of Leeds Sheeja Krishnan, Grant Lythe & Carmen Molina-Paris University of Manchester Rebecca Dearman, Amy Popple & Ian Kimber Salford Royal NHS Foundation Trust Jason Williams University College London Benny Chain & Theres Matjeka