* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lab 7_Esterification
Kinetic resolution wikipedia , lookup
Discodermolide wikipedia , lookup
George S. Hammond wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Sulfuric acid wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Petasis reaction wikipedia , lookup
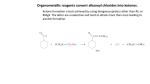
Experiment #7: Esterification Pre-lab: 1. Choose an ester to synthesize. Determine which alcohol and which carboxylic acid you will need to synthesize your ester. Write out the reaction for your specific synthesis in your notebook. Include the names along with the structures when you write out your reaction. 2. Find the molar mass and density of both your alcohol and your acid. Good resources are the Merck Index or chemfinder.com on the web. Calculate the mass and the volume that you will need for each. (It’s easier to measure volume with a liquid.) Make a reagent table in your notebook (just like the one below). You will finish filling in the last row after the experiment. 3. Prepare your notebook. Include: name, partner’s name, title of experiment, date, purpose, reaction scheme, reagent table, procedures (bulleted list), sketch of glassware set-up, and data. Leave spaces for observations. Format for reagent table: Molar Mass (g/mol) Density (g/mL) Mole equivalents Moles used Mass used (g) Carboxylic Acid Alcohol Ester * xxxxxxx * For products you record the expected values for 100% yield instead of amounts used. Volume used (mL) xxxxxxxxx Background: Esters, derivatives of carboxylic acids, are found in a variety of natural sources. Simple esters generally have pleasant odors, and are the main components of many of the odors and flavors of fruits and flowers. Most odors and flavors are the result of a complex mixture, with one ester predominating. Because esters are easy to synthesize, flavor chemists often use esters, either one or several, to reproduce or enhance natural flavors. The table on the next page shows some examples of esters that are primary components of various odors and flavors. You will choose one of these esters to synthesize. Although esters are often used as flavorings, they are rarely used as scents to be applied on the body. This is because esters are not highly stable, and can be hydrolyzed back to carboxylic acids and alcohols when exposed to perspiration. Since esterification is a reversible reaction, the addition of acid, water and heat (perspiration) favors hydrolysis. And, unfortunately, many simple carboxylic acids have a very unpleasant odor. For example, butyric acid (butanoic acid) is the main component responsible for the odor of rancid butter. Butyric acid is formed from hydrolysis of ethyl and methyl butyrate (butanoate), esters that smell like pineapple and apple. Some esters are also bioactive. Isoamyl acetate, which smells like bananas, is also an alarm pheromone released by honey bees when they sting an intruder. The pheromone attracts other bees and incites them to attack the intruder. For these reasons, fragrances are more often made from essential oils (phenols, aldehydes, alkenes, etc.). In this lab you will synthesize an ester from a carboxylic acid and an alcohol, and then purify it using extraction. You will choose which ester (see the list below) you want to make, and then choose the appropriate acid and alcohol for your synthesis. The general reaction scheme is shown below: O + R R' OH Cat. OH Acid O H2SO4 Alcohol R' R + H2O O Ester * Where R = alkyl group It is important to note that this is a reversible reaction, so the acid and alcohol reactants and ester products are in equilibrium (implied by the double arrow). What this means is that once the reaction reaches equilibrium, there could be a large amount of starting material remaining, resulting in a poor yield of the ester. In order to resolve this problem we make use of Le Chatelier's principle, which predicts that we can drive the equilibrium to the right (to the products) by having one of the reactants in excess (in effect, putting pressure on the left side). Usually, a threefold molar excess is enough to drive the equilibrium sufficiently to the right in an esterification reaction. Either the alcohol or the acid can be used in excess. The choice can be based on cost, availability and/or ease of purification at the end of the reaction. In this reaction we will add an excess of the acid. Sulfuric acid (H2SO4) is used as a catalyst for this reaction in order to accelerate the rate at which the product is formed. Since a catalyst is not consumed during the course of a reaction, you need to use only a small amount of sulfuric acid in order for it to be effective. Heating is another way to increase the reaction rate. We will heat the reaction using a reflux apparatus (see figure below). Refluxing is a process where liquid (your reaction mixture) is heated to its boiling point, with a condenser attached, so that some of the liquid vaporizes, rises part-way up the condenser, and then condenses and falls back down into the reaction flask. This process allows the reaction to be heated over a period of time, without evaporating away the solvent or reactants. At the end of the reaction your product will need to be purified, since it will be contaminated with unreacted acid (this is unavoidable because it was added in excess). The technique used for purification will be extraction using a separatory funnel. Extracting the product mixture with water and sodium bicarbonate (a base) will remove the carboxylic acid impurity. Some Esters and their Fragrances O Pear O (Propyl acetate) O Banana O (Isopentyl acetate) O O (Octyl acetate) Orange O O Peach (Benzyl acetate) O Rum O Isobutyl propionate) Materials: Alcohol (benzyl alcohol, isobutyl alcohol, isopentyl alcohol, octanol or 1propanol), carboxylic acid (acetic acid or propionoic acid), concentrated sulfuric acid, 5% sodium bicarbonate, anhydrous sodium sulfate, ether, methylene chloride, 25 mL round-bottom flask, water-cooled condenser, boiling stones, centrifuge tube with cap, transfer pipettes, test tube, small beaker, spatula. Safety Precautions: Concentrated sulfuric acid can cause extreme burns if spilled on skin or in eyes. Use with caution and wear safety goggles at all times. Ether is extremely flammable and alcohols are somewhat flammable and they should be kept away from flames. Flask will be hot at end of refluxing, allow glassware to cool before removing. Dichloromethane is not flammable, nor is it considered carcinogenic, but it can cause liver damage if ingested, or with repeated inhalation or skin exposures. It should be used in the fume hoods (inhalation of vapors can cause nausea or drowsiness). Avoid spills; it can also pass through both latex and nitrile gloves. Procedures: 1. Place your starting alcohol (0.03 mol) and your starting acid (0.09 mol) into a 25 mL round-bottom flask. Add 3-4 drops of concentrated sulfuric acid and mix by gentle swirling. Add a couple of boiling stones and attach the reflux condenser (see figure on previous page). Be sure to record the actual amounts and reagents used. 2. Start water circulating in the condenser (nice even flow), and bring the reaction mixture to a boil. Record the temperature. Continue heating under reflux for 40-60 minutes. When complete, remove the heating mantle and allow the mixture to cool to room temperature. 3. Disassemble the apparatus and transfer the reaction mixture to a centrifuge tube. Add 1 mL methylene chloride and shake to mix. Slowly add 3 mL of 5% sodium bicarbonate. Stir with a spatula until CO2 evolution is no longer vigorous. Then cap the vial and shake gently, with venting, until gas evolution is complete. Allow the layers to separate. The upper layer is the ‘organic’ phase and the lower layer is the aqueous phase. Using a pipette, remove the aqueous layer and discard. Repeat the extraction 2 more times (using 3 mL of 5% NaHCO3 each time). 4. Using a pipette, transfer the organic layer to a clean, dry large test tube. Using a spatula, add one or two scoops of anhydrous sodium sulfate. Allow to sit for 10 minutes, with occasional stirring. 5. Carefully decant into a clean, dry, pre-weighed small beaker (without transferring any of the solid). Evaporate the methylene chloride by flowing air over the surface. Do not heat. After all the methylene chloride is evaporated, note the odor and weigh the ester in the tube. Waste Disposal: The aqueous solutions should go into the aqueous waste beaker. Any excess acid, alcohol or methylene chloride should go into the organic waste container. The esters can be cleaned out with soap and water and go down the drain. Report: 1. Calculate the mass of your ester that was obtained (your yield). Calculate the theoretical yield for the reaction and the % yield (actual/theoretical x 100%). If needed, refer to Ch. 7 for how to calculate theoretical and % yields. 2. In your notebook, write a brief discussion of the experiment. In this section you should summarize your results (mass of ester obtained and % yield), note any interesting observations (odor and color of ester) and make any possible conclusions about the experiment (successful vs. unsuccessful and reasons why; 70% or higher is a good yield). 3. Answer the post-lab questions. 4. Make a copy of your lab notebook pages (do not tear out the originals) and attach your answers to the post-lab questions (if not in your notebook). Staple all pages and turn it in. Post-Lab Questions: 1. In this experiment we used both an acid catalyst and heat to increase the reaction rate. Briefly explain how each of these factors increases the reaction rate. Be specific, they each affect the rate in a different (yet related) way. 2. How would each of the following shift the equilibrium in the esterification reaction? a) Remove H2O as it is formed b) Add more H2SO4 catalyst 3. Why do we use an excess of the acid in this experiment? Give 2 reasons. (Hint: cost and availability are not factors, they are all relatively cheap and accessible.)