* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 20-2 Chemistry of Acyl Halides and Anhydrides(PPT)
Survey
Document related concepts
Transcript
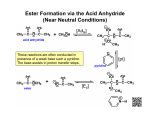
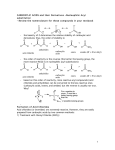
Organometallic reagents convert alkanoyl chlorides into ketones. Ketone formation is best achieved by using diorganocuprates rather than RLi or RMgX. The latter are unselective and tend to attack more than once leading to alcohol formation. Reduction of alkanoyl chlorides results in aldehydes. Alkanoyl chlorides are reduced to alcohols when sodium borohydride or lithium aluminum hydride are used directly. The reaction stops at the aldehyde if LiAlH4 is first reacted with three molecules of 2-methyl-2-propanol (tert-butyl alcohol), which reduces the nucleophilicity of the remaining hydride ion. 20-3 Chemistry of Carboxylic Anhydrides Carboxylic anhydrides are named by adding the term “anhydride” to the acid name (or names, if a mixed anhydride). This method also applies to cyclic derivatives. The reactions of anhydrides with nucleophiles are the same as for alkanoyl halide, only less vigorous. The leaving group is a carboxylate instead of a halide. Cyclic anhydrides undergo similar reactions which lead to ring opening. Alkanoyl halides are difficult to store for extended periods without undergoing hydrolysis from atmospheric moisture. Anhydrides, although less reactive towards nucleophiles, are more stable and many are commercially available. For these reasons, anhydrides are often preferred for the preparation of many carboxylic acid derivatives. Strong bases catalyze the hydrolysis of esters through an addition-elimination mechanism. The strong base converts the poor nucleophile H2O into the higher nucleophilic ion OH-. Unlike acid-catalyzed hydrolysis, base-catalyzed hydrolysis is driven to completion by the last step, which converts the carboxylic acid into a carboxylate ion. Ester hydrolysis is often carried out using the hydroxide ion itself, in at least stoichiometric amounts. Transesterfication takes place with alcohols. The direct conversion of one ester into another without proceeding through the free carboxylic acid can be carried out by reacting a second alcohol with an ester in the presence of strong acid. This process is called transesterification and is reversible. To shift the equilibrium, a large excess of the second alcohol is used. Lactones may be ring opened by transesterification. Acid-catalyzed transesterifications proceed by protonation of the carbonyl oxygen and subsequent attack by the alcohol. Base-catalyzed transesterifications proceed by deprotonation of the alcohol and subsequent attack at the carbonyl carbon. Amines convert esters into amides. Amines are more nucleophilic than alcohols. Esters readily transform into amides by treatment with an amine and subsequent heating. (A catalyst is not required.) Grignard reagents transform esters into alcohols. Two equivalents of a Grignard reagent will react with a normal ester to form a tertiary alcohol. In the case of a formate ester, a secondary alcohol is formed.