* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Transcript
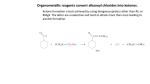
Did you know the aromas of the bananas, strawberries, and other fruits are the result of organic chemistry? Esters account for the distinctive odors of many fruits, Many of these ester compounds have pleasant odors. You can sy3~thesize an ester in the lab by reacting a carboxylic acid with an alcohol. You will need a catalyst to speed up the reaction. R--CO2H + R’--OH ~ R--CO2R’ + H20 carboxylic acid alcohol ester water In this experiment, you will react carbox’ylic acids and alcohols, in the presence of a strong acid catalyst, to form esters. Create esters from carboxylic acids and alcohols. safety goggles 5 medium test tubes 1 test-tube rack 1 ring stand 1 ring support 1 250-mL beaker l wire gauze 1 gas burner 1 dropper pipet 1 tbermonreter 1 10- mL graduated cylinder methanol, CHsOH [9 glacial ethanoic acid, ethanol, C2HsOH IT] 2-methyl- 1-propanol, C4HgOH ~ [] 1-pentanol, CsHIIOH 1-octanol, CaH~7OI}! 90% methanoic acid, c}[ coo salicylic acid, C~H4OHCOO H 18Msulfuric acid, H~SO4 ~T! distilled water coou Esters of Carbo)ylic Acids 303 Note the Safety Symbols used here and in the Procedure section. Review safety information on pages 7-9. Always wear safety goggles when working in the lab. All acids are corrosive, and several of the acids used in this experiment are concentrated and particularly hazardous. Alcohols are flammable liquids. Do not dispense these compounds near an open flame. MLx chemicals only according to directions. Never add water to 18Msul~ric acid. Never add 18M sulfuric acid to any other concentrated acid or to an alcohol. Asyou perform the experiment, record your obsmwations ii~ Table 50.1. 1. CAUTION: The acids used in this experime~tt are extremdy corrosive. Label five medium test tubes with the nnmbers 1-5. Put the robes in a test-tube rack. To each of the tubes, add 1 mL of a carboxylic acid and 1 mL of an alcohol, as listed in Table 50.1. In the case of the solid carb oxylic acid, salicylic acid, add 1 g of acid and 1 ml of alcohol to the tnbe. 2. CAUTION: Keep containers of alcohols and carbo.vylic acMs away from flames. Add 3 5 drops of concentrated sulfuric acid to each tube and heat the tubes in a water bath at 60°C for 10 15 minutes. a. Turn off the burner and remove the test tubes from the hot water bath, Cool the tubes in an ice bath. Add 5 mL of distilled water to each tube. Any ester produced in the reaction will float on the water in the tube. Note the odor of the ester by wafting the fl.maes toward your uose ~vith your hand. Try to relate each odor to a familiar odor of fruit, t]ower, or vegetable. Record your observations in Table 50,1. 4. Follow your teacher’s instructions for proper disposal of the materials. ame _ Class Date ~’able 50.1 Restflts aJld Conclusions C a r b o xylic Aci~=~’~’~ ..... ~coho’~l 1 2 3 4 5 methanoic acid glacial ethanoic acid glacial ethanoic acid glacial ethanoic acid salicylic acid 2-methyl- 1-propanol ethanol 1-pentanol 1-octanol methanol 1. Complete Table 50.1 by naming the esters that were synthesized in the experiment. 2. Write a general equation for the acid-catalyzed formation of an ester from an alcohol and a carboxylic acid. 3. Why was water added to tire tube before you were asked to smell it? (Hint: What was in the water layer in the test tube?) To answer this question, discnss the relative solubility of acids, alcohols, and esters in water. Esters of Carboxylic Acids 305 4. Wiry were the reactions kept h’ee of water during heating? 1. Write equations for each of tile esterificatiou reactions in this experiment. Use structural formulas in the equations and write the name of each compound below its structural fornmla. 2. In esterification reactions, the products are in chemical equilibrium with the reactants. Suggest several ways of causing the equilibrimn to shift in favor of tile production of additional ester. Data Table Test Tube # Organic Acid (20 drops) Alcohol (20 drops) 1 Salicyclic Methanol 2 Propionic Iso-butanol 3 Glacial Acetic Ethanol 4 Glacial Acetic 1-Pentanol 5 Glacial Acetic 1-Octanol Glacial Acetic 1-Propanol Name of Ester Formed Odor of Ester Formed