* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Adverse Reactions to Blood Products
DNA vaccination wikipedia , lookup
Immune system wikipedia , lookup
Innate immune system wikipedia , lookup
Anaphylaxis wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Adaptive immune system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Duffy antigen system wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Immunocontraception wikipedia , lookup
Rheumatic fever wikipedia , lookup
Food allergy wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Human leukocyte antigen wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
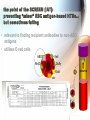
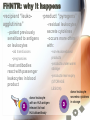
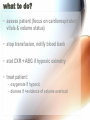
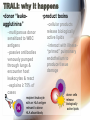
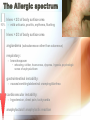
Adverse Reactions to Blood Products Medical Oncology Training Program Academic Half-Day Friday February 20th 2009, 11:00 am – 12:00 pm Christine Cserti-Gazdewich, MD FRCPC FASCP Assistant Professor, University of Toronto, Faculty of Medicine Transfusion Medicine/Hematology, University Health Network [email protected] UHN TGH BTL 3EC-306 14-6303 Reactions to Master…. • • • • • • • • • • • • hemolytic transfusion reactions FNHTRs allergic & anaphylactic reactions acute pain transfusion reactions bacterial contamination TRALI TAGVHD hypotensive transfusion reactions posttransfusion purpura stem cell reinfusion reactions circulatory overload IVIG adverse effects Reporting what how Our reporting obligations • WHAT: – all transfusion reactions (no matter how mild) and transfusion-related errors • TO WHOM: – hospital transfusion service • relays information to: – the Transfusion Transmitted Injuries Surveillance System (TTISS) at Public Health Agency of Canada – CBS or Héma-Québec if related to the quality of the product • DEPENDS UPON: – awareness & competence Transfusion Hazards Acute Delayed Immune Non-Immune Allergic TRALI BaCon AHTR / IBCT FNHTR DHTR HLA – PTR PTP TA-GVHD TACO Cold toxicity Citrate toxicity Hyperkalemia TTD Fe overload Deaths Due to Transfusion • # 1 cause of transfusion-attributed death: TRALI • overall risk of transfusion-related death: 1 in 200,000 chance = risk of death from anesthesia in a fit person Transfusion attributable symptoms, what they mean, & how they happen fever dyspnea allergy cytopenias delayed infections Fever HTR BaCon FNHTR when is it a fever? • T > 38ºC AND ↑ by Δ1ºC OR • the fever equivalent of chills or rigors Acute Hemolytic Transfusion Reaction • immune – active (recipient antibodies) – passive (donor antibodies) • non-immune – devices physically destroying RBCs – thermal injury to RBCs (hot/cold storage misadventure) – pressurized infusions – post-expiry infusions Acute Hemolytic Transfusion Reaction • active AHTR transfused allogeneic red blood cells D –recipient has, and can keep making, specific antibodies against a transfused cellular product that bears vulnerable antigens antibody-mediated hemolysis of transfused red cells D R host alloantibody attacks alloantigen Acute Hemolytic Transfusion Reaction high plasma-volume containing products (FFP, platelets) • passive AHTR –product carries donor antibodies which are specific for a recipient’s cells R antibody-mediated hemolysis of recipient red cells Causes of active AHTR • ABO mistake – failure to avoid anticipated ABO antibody – group O patient given non-O RBC * • anti-A and anti-B are naturally occurring antibodies and hemolysis is immediate • missed or hidden non-ABO antibody – failure to find unanticipated non-ABO antibody – use of emergency ‘uncrossmatched’ blood – antibody not detected on antibody screen * : ½ of these mistakes are due to hanging the properly labelled blood on the wrong patient, and many of the remainder are due to the recipient being wrongly characterized because of sampling errors the point of the SCREEN (IAT): preventing “minor” RBC antigen-based HTRs… but sometimes failing • relevant to finding recipient antibodies to non-ABO antigens • utilizes O red cells ABO Kell Rh(D) Duffy plasma Kidd etc 1 2 AHTR: clinical facts • presentation: fever/chills, hemoglobinuria, IV or flank pain, ↓BP, nausea/vomiting, dyspnea, renal failure, DIC • case fatality rate: <10% • non-morbidity rate: >50% • + “dose-response” risk (↑ blood = ↑ risk) • cases are usually ABO-related • non-ABO antigens implicated in 13% of AHTRs AHTR: How to pick it up… look for evidence of red cell incompatibility • clerical error search • re-type • direct antiglobulin test (DAT), aka Coomb’s test •assess plasma for hemolysis 10cc (or only 3% of a unit) of blood is visible…! direct Coomb’s test • re-screen or re-crossmatch • review labs indicative of hemolysis patient’s cells Suspected HTR: What to do Stop transfusion & run NS; check labels Notify blood bank, send post-transfusion specimen & remainder of product Stat lab work: acute renal failure work-up: electrolytes (hyperkalemia), creatinine DIC work-up: PT/INR, aPTT, fibrinogen Support BP & maintain good urine output Manage coagulopathy and bleeding Bacterial Contamination (BaCon) INCIDENCE • Risk of septic shock: – 1 in 10,000 PPC – 1 in 100,000 RBC • Risk of death from septic shock: – case fatality rate: 24-60% – 1 in 40,000 PLT pools – 1in 1,000,000 RBC • Key Message – Run transfusion slowly for 1st 15 min BaCon ETIOLOGY • donor bacteremia, skin plug, processing • most commonly: – Yersinia enterocolitica – Serratia marcescens/liquefaciens – Staph aureus/epidermidis PRESENTATION • fever, rigors, hypotension, renal failure, DIC • platelets more commonly implicated • RBC more severe FNHTR: what it is • = Febrile Non-Hemolytic Transfusion Reaction • a diagnosis of exclusion – after ruling out: • hemolytic transfusion reaction • bacterial contamination • underlying illness in patient • common – 1/10 platelet transfusions – 1/300 red cell transfusions FHNTR: why it happens •recipient “leukoagglutinins” –patient previously sensitized to antigens on leukocytes •product “pyrogens” –residual leukocytes secrete cytokines –occurs more often with: •old transfusions •pregnancies –host antibodies react with passenger leukocytes in blood product D donor leukocyte with an HLA antigen relevant to host HLA alloantibody •non-leukoreduced products •products under warm storage •products near expiry (STORAGE LESION) D donor leukocyte secretes cytokines in storage FNHTR: how to manage • acetaminophen • meperidine (Demerol) for rigors • PREVENTION? – acetaminophen • may not work • may obscure an important febrile cue to stop next transfusion – fresh components • not always available – plasma-reduced or washed products • risks product degradation/loss IVIG & Immune Reactions • immune-replacement / immune-modifying therapy = unique immune-mediated adverse effects, attributed to: – cytokines, vasoactive peptides, complement activation via Ig dimerization • common minor effects: – headache, fever, fatigue, chills, vomiting, nausea, dizziness, diarrhea, flushing, abdominal pain, chest tightness, cough, muscle cramps, pruritis, backpan • rarer, more serious effects: – sucrose-induced nephropathy, aseptic meningitis, hemolysis, anaphylaxis, vascular thrombosis Dyspnea TACO TRALI pulmonary allergic reaction (bronchospasm) what to do? • assess patient (focus on cardiorespiratory vitals & volume status) • stop transfusion, notify blood bank • stat CXR + ABG if hypoxic oximetry • treat patient: – oxygenate if hypoxic – diurese if +evidence of volume overload TACO • Transfusion-Associated Circulatory Overload • incidence: – 1 in 700 patients overall – 1 in 100 elderly recipients of blood • specific clues: – fast infusion rate – compensated chronic anemia (hyperdynamic before transfusion) – known congestive heart failure risk – orthopnea, ↑JVP, cyanosis, ↑HR, ↑BP TRALI: what it is • Transfusion Related Acute Lung Injury = NON-cardiogenic pulmonary edema • definition: – new acute lung injury (PaO2/FiO2 <300) – onset during or within 6h of transfusion – excludes: • TACO • underlying CHF • other pre-transfusion pulmonary morbidities • under-recognized & under-reported – estimates: 1 in 1200 to 1 in 5000 TRALI: why it happens •donor “leukoagglutinins” D •product toxins –multiparous donor sensitized to WBC antigens –passive antibodies venously pumped through lungs & encounter host leukocytes & react –explains ≥ 75% of cases H recipient leukocyte with an HLA antigen relevant to donor HLA alloantibody –cellular products release biologically active lipids –interact with illness“primed” pulmonary endothelium to produce tissue damage donor cells release biologically active lipids TRALI: outcomes • symptoms & signs: – dyspnea, cough, hypoxia, fever, BP ↑ or ↓, WBC ↓ • resolves in 24-72 hours • 72% require mechanical ventilation • death in 5-10% – most common cause of transfusion-attributed death now • supportive care is key – diuretic utility in non-cardiogenic edema? • prevention: – defer implicated donors – new mandates may restrict use of female plasma Allergy mucocutaneous vs multisystemic IgA deficiency & other causes The Allergic spectrum • hives < 2/3 of body surface area 90% – mild urticaria, pruritis, erythema, flushing • hives > 2/3 of body surface area • angioedema (subcutaneous rather than cutaneous) • respiratory: – bronchospasm • wheezing, stridor, hoarseness, dyspnea, hypoxia, psychologic sense of asphyxia/doom • gastrointestinal instability: – nausea/vomiting/abdominal cramping/diarrhea • cardiovascular instability: – hypotension, chest pain, tachycardia • anaphylactoid / anaphylactic reaction Why allergic reactions happen •Recipient-donor allergen-IgE interactions –classic allergic IgE antibodies and antigens, with one or the other passively infused and interacting in host •drugs, foods, chemicals –likely the most common explanation –no routine tests or traceback methods to confirm –URTICARIA to ANAPHYLAXIS •Recipient IgG sensitization to normal donor proteins –recipient has a rare polymorphism or deficiency of a certain protein compared to most donors •eg: IgA, haptoglobin, complement, albumin, α1anti-trypsin, transferrin –exposure to proteins = immunizing event –anti-protein IgG =ANAPHYLACTOID REACTION a few words on IgA deficiency • 1 in 500 are IgA deficient • 1 in 1200 have anti-IgA IgG (ie- proof of sensitization) – mysteries: • why/how IgG raised without known exposure • why ½ don’t react if exposed later – explains why reactions may be observed in a 1st transfusion How often & how severely they happen • within 1-45 min of transfusion, but up to 3 h afterwards • if severe: – as little as 10cc may be anaphylactic • dose-responsive • relapsing • frequencies: – minor: – severe allergic: – fatal anaphylaxis: 1/100 1 in 10 000 to 40 000 1 in 1 000 000 Management of Allergic Reactions • stop transfusion if >2/3rd BSA involved or noncutaneous (visceral) allergic symptoms • allergic anti-histamines – diphenhydramine (Benadryl), cetirizine (Reactine) ± H2R blockers – ranitidine (Zantac) • epinephrine, β2 receptor agonist bronchodilators • corticosteriods • ACLS: vasopressors, ventilatory support • future transfusions: – modify the patient: • premedication – modify the product: • ± plasma depletion / washing • obtain from donors deficient in the inciting allergen/antigen Hypotension HTR BaCon Allergy Bradykinin Bradykinin-mediated hypotension • bradykinin surge from contact activation: • for donors or recipients on ACE inhibitors: – reduced bradykinin breakdown from crossreactive suppression of enzyme from drug • recipients of bradykinin: – flushing, hypotension, dyspnea, hypoxia Cytopenias RBC (HTR) platelets (HLA PTR, PTP) WBC (TRAIN) pancytopenia (TA-GVHD) RBC decreases after transfusion • if immediate & not related to hemorrhage: – investigate for acute hemolytic transfusion reaction • usually related to ABO antibodies (preformed) & suspected from fever/hypotension • if delayed (3-14 days after transfusion): – investigate for delayed hemolytic transfusion reaction (DHTR) • usually non-ABO antibodies that took time to “resurge” after the offending red cell transfusion • may not have symptoms of hemolysis (fever, hemoglobinuria) – 1 in 6715 transfusions – in future: transfuse blood negative for antigen Platelet decreases after transfusion •HLA (human leukocyte antige) sensitization –recipient makes HLA antibodies from past exposure to WBCs •other transfusions •pregnancies •transplants –platelets express 73% of circulating HLA class I (A & B) antigens –recipient can destroy platelets bearing vulnerable HLA antigens –the cause of 80-90% of platelet transfusion refractoriness •HPA (human platelet alloantigen) sensitization –recipient with a polymorphic or missing platelet glycoprotein makes antibody against the common glycoprotein type •pregnancies •other transfusions –recipient can destroy platelets bearing vulnerable HPA antigens –leads to PTP (posttransfusion purpura) –fatal in 8% –fortunately RARE! Platelet-Specific Antigens in PTP Most frequently involved polymorphism (75% of PTP cases) But fortunately only 28% make the antibody if exposed Platelet Antigen System Protein Antigen Alleles Antigen Frequency HPA-1 GPIIIa HPA-1a = PlA1 HPA-1b = PlA2 97% 3% HPA-2 GPIb HPA-2A HPA-2b 99% 14% HPA-3 GPIIb HPA-3a HPA-3b 85% 66% HPA-4 GPIIa HPA-4a HPA-4b >99% <1% HPA-5 GPIa HPA-5a HPA-5b 99% 20% Post transfusion neutropenia •donor anti-granulocyte antibodies –although usually implicated in TRALI, may also (or instead) cause neutropenia of host’s neutrophils –passive alloimmune reaction TA-GVHD • Transfusion-Associated Graft Versus Host Disease • passenger lymphocytes “engraft” in recipient – who: • immune compromised • immune recognition failure – what happens: • bone marrow aplasia (empyting from attack) = pancytopenia (all counts down) • fever, rash • liver dysfunction, diarrhea • death in 90% Vulnerable recipients •immune compromised –congenital immunodeficiency –fetuses, premature neonates –hematologic malignancies (eg lymphoma) –stem cell transplants –high dose chemotherapies with immune-ablation potential –nucleoside analogue drugs (eg fludarabine for chronic lymphocytic leukemia) •immune “oblivious” –host’s HLA type is foreign to transfused lymphocytes, which resemble the host enough to be tolerated •eg. donor is HLA “homozygous haploidentical” with respect to recipient •related donors •1 in 18 000 – 39 000 of strangers A1 A2 B7 B9 C4 C8 recipient tissue type A2 A2 B9 B9 C8 C8 donor lymphocyte Delayed onset infections Viruses (H A/B/C V, HIV, CMV, HTLV, WNV, PVB19, HHV8) Prions (vCJD) Others (protozoal, helminthic, spirochetal, rickettsial) Viral testing of each donation • all donors: – – – – – – HIV Ab, HIV NAT HCV Ab, HCV NAT HBsAg, HBcAb Syphilis HTLV-1/2 Ab WNV NAT • selected cases/products: – CMV Ab – bacterial culture (pre-pooled/high volume platelets only) – Parvovirus B19 (fractionated products only) Residual risk (window period misses) Virus Risk per donor exposure Outcome of exposure HIV 1 in 4.7 million 50% risk of HIV in 7y, shorter latency in elderly HCV 1 in 3.1 million 50-70% chronic hepatitis HBV 1 in 82 000 60% hepatitis with <5% chronicity HTLV 1 in 3 million unknown WNV <1 in 1 million at least 20% Other transfusion transmissible infections •Malaria •Toxoplasmosis •Leishmaniasis •Filariasis •Ehrlichiosis •Babesiosis •Trypanasoma cruzi (Chagas) •Borrelia burgdorferi •Rocky mountain spotted fever (rickettsial group) •Q fever (Coxiella burnetti) •variant CJD at this time, screening by donor history alone is performed in order to prevent the entry of these infections in the donor supply