* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ronald van Ree
Molecular mimicry wikipedia , lookup
Immune system wikipedia , lookup
Innate immune system wikipedia , lookup
Complement system wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Adaptive immune system wikipedia , lookup
Immunocontraception wikipedia , lookup
Gluten immunochemistry wikipedia , lookup
Anaphylaxis wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
DNA vaccination wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Autoimmune encephalitis wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
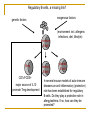
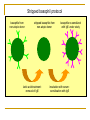
Mechanisms of sensitization, disease development and desensitization: towards novel approaches for prevention and therapy Ronald van Ree Academic Medical Center, Amsterdam Priorities for allergy/asthma research Immunology: B-cells and antibodies Immunotherapy: mechanisms and monitoring Atopic march: eczema → asthma → rhinitis → asthma? B-cells and antibodies Interaction between allergen and IgE is at the basis of allergy and (extrinsic) asthma. Exposure to allergen results in: no response? protective response (“active tolerance”)? sensitization? +/- symptoms? How is the process of B-cell activity towards or away from allergic inflammation regulated? genetic factors exogenous factors (environment incl. allergens infections, diet, lifestyle) DC naïve Th Th2 IFNγ Th1 no allergy IL4/IL13 Th2 Th2 Th1 Ig? B-cell Th1 IgM B-cell IgE B-cell allergy genetic factors exogenous factors (environment incl. allergens infections, diet, lifestyle) DC Scheme of a simple Th1-Th2 disbalance to distinguish between allergic and non-allergic has actually been dismissed as being too simple. naïve Th Th2 IFNγ Th1 no allergy IL4/IL13 Th2 Th2 Th1 Ig? B-cell Th1 IgM B-cell IgE B-cell allergy genetic factors exogenous factors (environment incl. allergens infections, diet, lifestyle) DC naïve Th Treg Th1 ? no antibody response? IgG? IgA? Th2 IFNγ IL4/IL13 Th1 IL10 and/or TGFβ Treg IgG/A? B-cell no allergy healthy non-atopic? Th1 Th2 Th2 IgM B-cell IgE B-cell allergy genetic factors Questions and Discrepancies exogenous factors • healthy immune response against allergens: Treg/IL10/TGFβ or Th1/IFNγ or both? • do B-cells of non-atopic individuals ignore allergens or(environment produce IgG/IgA incl. antibodies? allergens • Is this different for low-exposure allergens (e.g. pollen and mite) and highinfections, diet, lifestyle) exposure allergens (e.g. cat, bee venom and occupational allergens)? DC naïve Th Treg Th1 ? no antibody response? IgG? IgA? Th2 IFNγ IL4/IL13 Th1 IL10 and/or TGFβ Treg IgG/A? B-cell no allergy healthy non-atopic? Th1 Th2 Th2 IgM B-cell IgE B-cell allergy genetic factors exogenous factors timing/dose/context of allergen exposure DC Th1 naïve Th modTh2 IgE? B-cell IgG4 B-cell no allergy IgG1 B-cell Th1 IL4/IL13 Th2 Th2 modTh2 IL10 IgM B-cell IgE B-cell allergy healthy atopic (or atopic treated by immunotherapy) and non-atopic? genetic factors exogenous factors Questions and Discrepancies • is a modified Th2 response in atopic individuals IgG4 without IgE or with (little/harmless) IgE? of Th2? • is there an early-life window of opportunity for inducingtiming/dose/context the protective modified allergen exposure • why are IgG4 responses to allergens higher in atopics than in non-atopics? • atopic: predisposition to produce IgE and high IgG4? non-atopic: no tendency to develop IgE and only low IgG4? DC • atopic background seems to be less important for IgE/IgG4 production in case of high exposures: cats, bee venom, occupational allergens, parasites. Th1 naïve Th modTh2 IgE? B-cell IgG4 B-cell no allergy IgG1 B-cell Th1 IL4/IL13 Th2 Th2 modTh2 IL10 IgM B-cell IgE B-cell allergy healthy atopic (or atopic treated by immunotherapy) and non-atopic? High early exposure to cat allergen is protective Allergy (sensitization) IgG (exposure / protection) Increased exposure to cat allergen Lancet 2001 ; Tom Platts-Mills et al. Why are IgE responses always so low compared to IgG responses? Half-life of IgE is very short but this can not explain the 1000-fold difference in serum titers. A major cause most likely is the poor generation of memory B-cells for IgE caused by inefficient processing of mRNA for membrane IgE. Circulating IgE is derived from long-lived plasma cells hiding in survival niches like the bone-marrow and inflammatory sites. Two situations: • low allergen exposure (e.g. pollen/mite) i.e. a weak Th2 response that will not effectively induce a germinal center necessary for induction of memory B-cells • high allergen exposure (e.g. cat, bee venom) i.e. a strong Th2 response that will a generate mature germinal center facilitating induction of memory From: Aalberse RC et al. J Allergy Clin Immunol. 2004 May;113(5):983-6. Low exposure situation: no memory only plasma cells plasma cells Atopics respond with all three but IgG is not protective under these conditions Non-atopics only make a little IgG / are hypo-responsive overall compared to atopics. From: Aalberse RC et al. J Allergy Clin Immunol. 2004 May;113(5):983-6. High exposure situation: memory generation for IgG but not for IgE plasma cells Poor expression of membrane form of IgE favors apoptosis resulting in poor memory This also reflects the situation during allergen-specific immunotherapy For primary prevention it is of the greatest importance to investigate the dose-response relation between allergen and (the quality) of the immune response. The window of opportunity is of great importance. A high-dose protective effect as observed for cat has so far not been found for house dust mite. For food allergens this is even more debated. The outcome has very significant public health impact. “Promote cats and peanut butter sandwiches early on or not?” What is needed to study the dose-response relation between allergen exposure and (the quality of) the immune response? • Analysis of (existing) birth cohort samples for IgG and IgA responses. In most cases only IgE has been measured. Multiple time points in first year of life are required. Multiplex systems now allow in detailed analysis with limited sample. • Analysis of (existing) birth cohort samples for mRNA profiling • In vitro cellular analyses to study the process of immune skewing, preferably in an allergen specific fashion. • Mouse models that go beyond the ovalbumin model by using real allergens. Regulatory B-cells, a missing link? exogenous factors genetic factors (environment incl. allergens infections, diet, lifestyle) immature B-cell Breg CD1dhiCD5+ major source of IL10 promote Treg development memory B-cell plasma B-cell In several mouse models of auto-immune diseases an anti-inflammatory (protective) role has been established for regulatory B-cells. Do they play a protective role in allergy/asthma. If so, how can they be promoted? The quality of IgE antibodies Do they always translate into clinical allergy? No There are several examples of IgE responses that are without clinical relevance, i.e. the ones observed during parasite infections, the ones directed to plant glycans and the ones observed during successful immunotherapy. Tregs or Th1 cells can not explain the lack of biological activity. The explanation can also not (always) simply be found in a protective effect of IgG4 (“blocking antibodies”). How is the biological activity of IgE assessed? Stripped basophil protocol basophils from non-atopic donor stripped basophils from non-atopic donor lactic acid treatment: removal of IgE basophils re-sensitized with IgE under study incubation with serum: sensitization with IgE Allergen-specific IgE from parasite-infected children lacks activity. This is not explained by high allergen-specific IgG4 titers specific IgE Gabonese (pink) vs Dutch (green) (n=16) (n=7) 35 % histamine release (SE) mean (SE) of anti-mite IgE in IU/ml 40 30 25 20 15 10 5 80 60 a-IgE mite 40 a-IgE mite 20 0 0 0.1 Gabonese Dutch 10 ng/ml What explains the poor biological activity? 1000 IgE antibodies against plant N-glycans have the same poor biological activity % histamin release 60 transgenic human lactoferrin 40 native human lactoferrin 20 rPhl p 5 0 0.0001 0.01 1 100 10000 concentration (ug/ml) An explanation needs to be found why some IgE antibodies are poor inducers of mediator release, i.e. without clinical relevance. What is different about these type of IgE antibodies? Lower affinity? Something else? Is this also observed during immunotherapy where IgE antibodies are persistent but skin reactivity clearly decreases. Are these IgE antibodies the same as before the start of therapy or are they (partly) newly formed IgE antibodies. B-cells and antibodies have not received the attention they deserve compared to T-cells. They do however produce the antibodies that cause the disease!! Reasons to study IgE/IgG/IgA antibody responses: • They are a good read-out of the nature of the immune response • It needs to be established when/why they are clinically relevant, i.e. either disease-inducing (IgE) or protective (IgG/IgA). • Is induction of IgG/IgA before IgE a protection against future sensitization? • What is the difference between IgE from direct (μ to ε) and from indirect (μ to ε via γ4)? Reasons for studying B-cells: • What determines the quality of IgE antibodies? • What is the role of Bregs in allergy/asthma? • How could we target persistent plasma B-cells for IgE? Immunotherapy: mechanism and monitoring Many of the same questions need to be answered. Blocking IgG4 antibodies? What is the difference between IgE before and after therapy? Th1 skewing? Tregs? Or perhaps Bregs? Expression profiling / proteomics for biomarker search Investigator-initiated trials needed. Companies are too small to set up trials that include in depth immunological analyses. Better insight in mechanism is a prerequisite for improvement of therapy. Primary outcome (natural exposure) is an ill-controlled disaster! Towards pollen chamber monitoring in season.