* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Previously in Cell Bio
Endocannabinoid system wikipedia , lookup
Drug design wikipedia , lookup
Lipid signaling wikipedia , lookup
Interactome wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Proteolysis wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Biochemical cascade wikipedia , lookup
Western blot wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Ligand binding assay wikipedia , lookup
Paracrine signalling wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
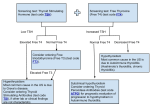
Previously in Cell Bio Signals are detected via binding interactions Binding interactions governed by protein folding Protein folding dictated by amino acid sequence (molecular models as link from index page) Hypotheses for ‘problem’ in Graves’ Disease Positive signals (TRH or TSH) altered to increase amount or affinity for their receptor Signal receptor altered to increase their affinity for hormone Problem with signal relay ‘inside’ thyroid Graves’ hypothesis 1: TSH, TSH-Receptor interaction ‘too strong’ According to this hypothesis and what we now know about protein binding…… T3 and T4 levels should be _?_ in Graves’ vs. normal. TSH levels should be __?__ in Graves’ vs. normal TSH/TSH receptor interactions should show __?___ binding constant vs. normal. Blood tests show T3 and T4 levels are elevated TSH levels are decreased TSH/TSH receptor interactions have same binding constant vs. normal. Therefore: Perfectly logical hypothesis……. Not supported by data Now what? Hypothesis 2: Mutation in signaling within cell leading to increase in thyroid hormone production Normal activation is the result of signal transduction second messenger cascade How does signal transduction work? What could have gone wrong? What do we know so far? •Thyroid is ‘overacting’ •Pituitary normally responsible for thyroid stimulation through levels of TSH •Graves’ patients have normal/decreased levels of TSH in blood •Binding affinity between TSH and TSH-R normal More of what we know •TSH is water soluble hormone (why is this important?) Figure 4-1. Schematic drawing of human TSH, based on a molecular homology model built on the template of a hCG model14. The a-subunit is shown as checkered, and the b-subunit as a solid line. The two hairpin loops in each subunit are marked L1, L3; each subunit has also a long loop (L2), which extends from the opposite site of the central cystine knot. The functionally important a-subunit domains are boxed. Important domains of the b-subunit are marked directly within the line drawing (crossed line, beaded line and dashed line): For further details the reader is referred to Grossman et al.2. (Reproduced from Grossman,M, Weintraub BD, SzkudlinskiMWEndocrin Rev (4) 18:476501,1997, with permission of the Endocrine Society). From “The Thyroid manager” •Thyroid Evenplasma moremembrane is barrier to polar molecules •TSH interacts with a receptor on the surface of thyroid cells HOW and WHY is the thyroid responding as though over-stimulated? And to get to the answer of that question: How do signals get passed across membranes? Characteristics of Transmembrane Proteins •Hydrophobic face of protein in transmembrane region -one continuous structure or multiple regions of 2° structure •Charges ‘anchor’ transmembrane region •Asymmetric orientation Peripheral Membrane proteins Characteristics •Associations with membrane not as strong •Various means of attachment -Protein-protein -Protein-phospholipid head Fig 3-32 Molecular Cell Biology by Lodish et al. Membranes and membrane proteins How can a polar signal gain access to the cytosol Direct access: From the ‘outside’ •Pores •Channels •Pumps From cytosol to cytosol •Gap junctions Membrane proteins Indirect access: Receptors If signaling molecule never gains access to cytosol how can the information be transmitted? Extracellular domain Plasma Membrane Cytoplasmic Domain TSH Receptor: from “The Thyroid Manager” Ch16 Transmembrane receptors •Same general structure as other transmembrane proteins •Able to bind specific ligand •Ligand binding causes conformational change What change in the TSH receptor could cause overproduction of T3 and T4 How could you test your hypothesis? Allosteric transitions What are they, why are they important, How do they relate to signal transduction •R T state transitions •Cooperative binding Examples DNA helicase and ras (links from index page) Other mechanisms that regulate protein function •Compartmentalization •Change in rate of synthesis Common traits? •Cleavage •Phosphorylation/dephosphorylation Common traits? Receptor’s role (summary) Able to transduce signal because of: •Placement in membrane (span it) •Ability to bind ligand •Ligand -induced conformational changes So the signal ‘gets in’ without physically crossing membrane BUT How do you go from a shape change to causing a change in gene expression? 2nd Messengers and Signaling Cascades Getting the signal to where it needs to go