* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 9Calcium AP
Signal transduction wikipedia , lookup
Chemical synapse wikipedia , lookup
Cyclic nucleotide–gated ion channel wikipedia , lookup
Cell encapsulation wikipedia , lookup
Node of Ranvier wikipedia , lookup
List of types of proteins wikipedia , lookup
Cell membrane wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Endomembrane system wikipedia , lookup
Action potential wikipedia , lookup
Membrane potential wikipedia , lookup
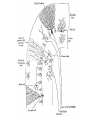
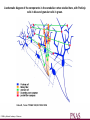
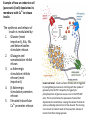
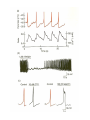
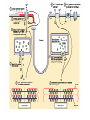
Calcium-Dependent (-Derived) Action Potentials Two Types 1. Na+/Ca2+-dependent APs. • Present in axon terminals, cardiac myocytes and skeletal myocytes (for muscle fiber contraction). • Occurs in response to neurotransmitter release in the presynaptic axon. Two Types (cont’d) 2. Ca2+-dependent AP (No Na+). Present in dendrites of Purkinje cells (cerebellum) and in endocrine cells to trigger hormone secretion. Characterized by small amplitude and long duration. A schematic diagram of the components in the cerebellar cortex studied here, with Purkinje cells in blue and granular cells in green. Cohen D , Yarom Y PNAS 1998;95:15032-15036 ©1998 by National Academy of Sciences Example of how an endocrine cell (pancreatic β-cell) depolarizes its membrane with Ca2+ to release insulin. The synthesis and release of insulin is modulated by: 1. Glucose (most important), AAs, FAs and ketone bodies stimulate release. 2. Glucagon and somatostation inhibit relases 3. α-Adrenergic stimulation inhibits release (most important). 4. β-Adrenergic stimulation promotes release. 5. Elevated intracellular Ca2+ promotes release. Insulin secretion - Insulin secretion in beta cells is triggered by rising blood glucose levels. Starting with the uptake of glucose by the GLUT2 transporter, the glycolytic phosphorylation of glucose causes a rise in the ATP:ADP ratio. This rise inactivates the potassium channel that depolarizes the membrane, causing the calcium channel to open up allowing calcium ions to flow inward. The ensuing rise in levels of calcium leads to the exocytotic release of insulin from their storage granule. Why are fibers of the conducting system autorhythmic? If channels How does the depolarization in these cells affect cardiac muscle cells? Superimpose changes in the muscle cell’s membrane potential on this graph Membrane potential of SA nodal cells Ventricular AP •Phase 4: resting membrane potential near the K+ equilibrium potential. •Phase 0: depolarizing impulse activates fast Na+ channels and inactivates K+ channels. •Phase 1: Transient opening of K+ channels and Na+ channels begin to close. •Phase 2: Ca2+ channels are open, key difference between nerve AP. •Phase 3: repolarization, Ca2+ inactivate and K+ channels open. •Refractory period: Na+ channels are inactive until membrane is repolarized. Changes in ion concentrations in a cardiac muscle fiber following depolarization What causes the muscle resting membrane potential to change initially? What would be happening with a skeletal muscle at this point? Similarities • Note that whatever cation generates the AP, all APs share a similar structure and and all are activated by membrane depolarization. • Thus, the Na+, Na+/Ca2+ and Ca2+ APs all have a similar pattern of depolarization – influx of some cation. • Repolarization all entail the inactivation of Na+ or Ca2+ channels together with K+ efflux. Differences Na+/K+ Ca2+ • Threshold lower; therefore, depolarization sudden. • AP decays with distance (but, do you recall what keeps it going over long distances?). • Depolarization: Na+ channels open nearly simultaneously. • Threshold higher and gradual; therefore depolarization gradual. • AP does not attenuate over distance – what keeps it going? (syncytium, gap junctions, highly branched processes). • Local, gradual, and transient Ca2+ entry – allows for fine control and adjustments. Propagation of Electrical Signals in Heart Muscle • Heart muscle is syncytial