* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Current Therapy of Genetic Disorders
Gene expression programming wikipedia , lookup
Point mutation wikipedia , lookup
History of genetic engineering wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Genome editing wikipedia , lookup
Tay–Sachs disease wikipedia , lookup
Genetic engineering wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Microevolution wikipedia , lookup
Medical genetics wikipedia , lookup
Genome (book) wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Public health genomics wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene therapy wikipedia , lookup
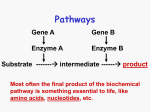
Current Therapy of Genetic Disorders • • • • • Preventive Metabolic Manipulation Gene Product Replacement Cell or Organ Transplantation Gene Therapy Therapy of Genetic Disorders • Preventive Therapy – Prenatal diagnosis – Preimplantation diagnosis (in vitro fertilization, testing of embryo & implantation of normal embryo) – Preventive screening for disease onset Therapy of Genetic Disorders • Metabolic Manipulation – Dietary restriction • (Lactose restriction for Lactase deficiency; phenylalanine restriction for phenylketonuria) – Dietary Supplementation • (Vitamin C for Scurvy, Biotin for Biotinidase deficiency, Starch for G-6-P deficiency) – Chelation and enhanced excretion • (copper chelation for Wilson Disease) – Metabolic inhibitors • (allopurinol for gout, Statins for hypercholesterolemia,) Lactase deficiency: Dietary Restriction: lactose free (dairy products) Dietary supplementation: lactase pills All other mammals and most people lose the ability to digest lactose by adulthood Lactase persistence is found in 50-90% of Europeans but is much rarer in other populations Lactase persistence is associated with two single nucleotide polymorphisms (SNPs) 5’ of LCT -13910 C/T, -22018 G/A Lactase has been under very strong selection + √ Perhaps? Persistent LCT ???? Estimated age of lactase persistence haplotype 180 generations (3,600 years) Dairy farming: 5,000-9,000 years ago Courtesy JN Hirschhorn Harvard ?Evolutionary cure of lactase deficiency • Genetic signatures of recent positive selection can be found • LCT (lactase) shows remarkably strong evidence of recent positive selection • New metrics may locate other regions of the genome that have been under recent positive selection Courtesy JN Hirschhorn Harvard • December 2006: Reports of additional mutations leading to persistence of lactase in Africa in different dairy farming regions • With signatures of strong positive selection • ?Nature, Tischkof et al Dietary Supplementation for Biotinidase deficiency: Biotin Cycle Symptom >50% Alopecia (hair loss) Developmental delay Hypotonia (poor muscle tone) Ketolactic aciduria Seizures Skin rash/skin infection 2550% Ataxia (poor coordination) Conjunctivitis (redness of the eye) Hearing loss Lethargy (drowsiness) Mild hyperammonemia Breathing problems Eye problems 1025% Coma Feeding difficulties/vomiting/diarrhea Fungal infections <10% Hepatomegaly (enlarged liver) Speech problems Splenomegaly (enlarged spleen) Effects of Dietary SupplementationTherapy with Oral Pharmacologic Doses of Biotin Before Biotin treatment After Biotin treatment Therapy of Genetic Disorders • Gene Product Therapy – Hormone, protein or enzyme replacement • Hormone supplementation: – Hypothyroidism: thyroid – Congenital adrenal hyperplasia: cortisol – Growth hormone • Hemophilia; clotting factors • Diabetes: insulin • Enzyme replacement – Beta glucosidase : Gauchers – Alpha glucosidase: Pompe – Adenosine deaminase (PEG): ADA- SCID Enzyme Replacement Therapy of Inherited Disorders • • • Extension of paradigm of therapy for deficiency of plasma proteins (eg hormones & clotting factors) May not require targeting intracellularly if stored or toxic metabolite is in equilibrium with plasma Targeting of deficient protein into cells and organelles would provide the widest application Examples of Current Enzyme Therapy • Current FDA approved enzyme replacement therapy – Adenosine deaminase deficiency (SCID)• Severe combined Immunodeficiency • No targeting to cells, but removal of metabolites from plasma – Several Lysosomal Storage Disorders • Genetic deficiency of Lysosomal Enzymes • Therapy: Targeting of deficient enzyme to lysosomes Adenosine deaminase deficiency: cellular & metabolic interactions lymphoid cells & RBCs other cells dATP nl dATP deoxyadenosine Adenosine deaminase deficiency: cellular & metabolic interactions: Effect of enzyme therapy lymphoid cells & RBCs dATP deoxy adenosine Injection of PEG Calf Adenosine Deaminase deoxyinosine Lysosomal Storage Diseases • Lysosomes: intracellular organelles containing hydrolytic enzymes that degrade macromolecules (recycling and “garbage disposal” for cells) • Lysosomal enzymes are targeted to lysosomes by interaction with Mannose 6 PO4 receptors in the cell • Lysosomal enzymes can be taken up into the cell from plasma by interaction with Mannose 6 PO4 receptors on cell surface ENZYME REPLACEMENT THERAPY FOR LYSOSOMAL STORAGE DISEASES Disease Current Status • Gaucher Disease Approved 1991 • Fabry Disease Approved 2001/03 • Mucopolysaccharidosis I Approved 2003 • Mucopolysaccharidosis VI Approved 2005 • Mucopolysaccharidosis II Approved 2006 • Pompe Disease Approved 2006 • Niemann-Pick B Disease Phase 1 Trial Therapy of Genetic Disorders • Cell or Organ Transplantation Cells Bone marrow Immunodeficiency Disorders Organs Kidney Fabry Disease Liver Tyrosinemia Gene Therapy: Types • Introduction of normal gene – Somatic – Germ line • Therapy of noninherited disorders – (cancer, AIDS) • Production of gene product for administration – (hemophilia, growth hormone, erythropoietin) Somatic Gene Therapy Introduction of recombinant genes into somatic cells to treat genetic or acquired disease • Does not involve germ line • applicable to any disease with known molecular basis of pathogenesis • currently does not involve removal, repair or site-specific replacement of mutant genes • may not require permanent alteration of cells (repetitive therapy) Disease Characteristics Currently Ideal for Gene Therapy • • • • • • • Lethal disorder Course not highly variable Reversible No universal therapy Gene cloned No tissue specificity or regulation Bone marrow cells involved State of the Art of Genetic Engineering • Ideal – Replace defective gene with normal (site specific insertion) – Target vector containing the gene to damaged cell – In vivo administration safe, effective and permanent (integration into DNA but not at oncogenic sites) – Vector contains all regulatory elements • Current – Site specific insertion very early and experimental – No current trial incorporates all of the ideal requirements Gene Therapy Potential Successes Disease Cell/tissue Vector X-linked & ADASevere Combined Immunodeficiency Stem Cell (bone marrow) Retrovirus Somatic Mosaicism: Reversion of an Inherited Mutation to Normal and Selective Growth Advantage Mutant Reversion of mutation to normal Mutant cells Normal cells “Successful” Gene Therapy for Immunodeficiency Diseases:2005 • Retroviral vector used despite major disadvantages • Over 14 patients with X linked severe combined immunodeficiency of 3 different types have been treated successfully • Oncogenic insertion in two of 14 children-leukemia • - X-linked SCID trials suspended but now reinstituted • ~8 patients with ADA deficiency treated Current Therapy of Genetic Disorders • • • • • Preventive Metabolic Manipulation Gene Product Replacement Cell or Organ Replacement Gene Therapy Current Therapy of Genetic Disorders • Experimental • Gene Therapy (Experimental) • Correction with oligonucleotide or RNAi • Silencing (RNAi and others) • Transplicing • “Read through” of nonsense mutations Ideal Viral Vectors • • • • • Replication defective Accommodates large inserts High titer with broad cell range High level of expression of inserted gene Unique promotors – Tissue specific vs universal – On/off switch; controllable expression • Non-toxic Current Enzyme Therapy of Lysosomal Disorders with Intracellular Replacement of Enzyme: Currently “standard of care” Gauchers Disease (beta glucosidase; non neuronopathic) Current Clinical Trials: Glycogen Storage Disease Type II (acid maltase) Fabry Disease (alpha galactosidase) Hurler Disease (alpha iduronidase) Hunter Disease (iduronate sulfatase) Lysosomes Lysosomal enzymes contain a recognition site for targeted uptake into cells and lysosomes • enzyme protein synthesized in endoplasmic reticulum • carbohydrate (high mannose) added in ER • • mannose 6 phosphate added in Golgi: some enzyme secreted to outside of cell mannose 6 phosphate binds to receptors, leading to targeting to lysosomes and uptake of enzyme into cells Enzyme Replacement Therapy for Lysosomal Disorders • Requirements – Unprocessed enzyme that will be taken up by cells and targeted to lysosomes • Solutions – Purify from cultured cells • (introduce gene into cells, amplify copy number, mass culture, purify protein) Currently FDA approved approach – Purify from milk of an animal • (attach casein promotor to gene, introduce into ovum (transgenic), select animal(s) producing greatest amount of enzyme, purify enzyme from milk) Attractive but problems and difficult to get approval Considerations for Gene Therapy • State of the art of genetic engineering • State of the art of manipulation of cells and organs • Disease characteristics Variables in Current Gene Therapy Trials • Vector for delivery of gene • Ex vivo vs In vivo administration • Permanent integration into DNA vs transient expression • Incorporation of regulatory elements Examples of Current Enzyme Therapy • Current FDA approved enzyme replacement therapy – Adenosine deaminase deficiency (SCID)• Severe combined Immunodeficiency • No targeting to cells, but removal of metabolites from plasma – Gaucher Disease (Beta glucosidase deficiency) • Gaucher Disease (non-neuronal only) • Lysosomal Storage Disorder • Targeting of deficient enzyme to lysosomes Types of Somatic Gene Transfer • Ex vivo – Gene or expression vector carrying the gene is inserted into explanted or cultured cells which are then transplanted into the patient • In vivo – Gene or expression vector carrying the gene is administered directly to the patient ENZYME REPLACEMENT THERAPY FOR LYSOSOMAL STORAGE DISEASES Gaucher Disease Fabry Disease Approved 1991 Approved 2001 (EU), 2003 (US) Mucopolysaccharidosis I Approved 2003 (EU & US) Mucopolysaccharidosis VI Approved, 2005 (US& EU) Mucopolysaccharidosis II Approved, 2006 (US) Pompe Disease (AMD) Niemann-Pick B Disease Approved, 2006 (US & EU) Phase 1 Trial Underway