* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download MCB_151_Exercise 10_Glow
Molecular cloning wikipedia , lookup
Gene regulatory network wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Molecular evolution wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Genomic imprinting wikipedia , lookup
Community fingerprinting wikipedia , lookup
Ridge (biology) wikipedia , lookup
Genome evolution wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Genomic library wikipedia , lookup
Genetic engineering wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Gene expression profiling wikipedia , lookup
Transformation (genetics) wikipedia , lookup
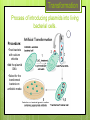
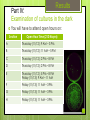
Producing a Strain of E.coli that Glows in the Dark Exercise 10 Announcements Post Lab 10 and Pre Lab 11 is due by your next lab LNA Glow Lab is assigned today, and is due next week in lab Exam II is during your lab period the week of November 30 and will cover Exercises 8-11 The Final Exam is scheduled for Friday, December 11 from 8:00-11:00 AM. Locations TBA. If this conflicts with any of your other final exams, you must fill out the Final Exam Conflict Request Form by 5 PM on 11/9/15. Goals To create an ampicillin-resistant population of E. coli by introducing a plasmid that contains an ampicillin resistance gene Understand the lux Operon and how it is used to create luminescent bacteria Understand the pUC18 plasmid and how it serves as a control in this experiment Penicillin One of the most important anti-infective agents used in clinical medicine Interferes with bacterial cell wall synthesis Over 500 semi-synthetic penicillins have been made during the past 30 years Plasmids Plasmids are small circular DNA molecules that exist apart from the chromosomes in most bacterial species Many contain genes that enable bacteria to survive and prosper in certain environments b/c they carry genes that provide resistance to antibiotics pUC18 Plasmid Circular DNA that contains 2686 nucleotides (very small) Contains an ampicillin-resistance gene pUC18 Plasmid Transformation Process of introducing plasmids into living bacterial cells. Procedure: •Treat bacteria with calcium chloride •Add the plasmid DNA •Select for the transformed bacteria on antibiotic media lux Operon Contains two genes that code for luciferase and several genes that code for enzymes which produce luciferins R T C D A B E G Rib The lux Operon. The lux operon contains two genes for the luciferase enzyme (A and B). This enzyme is composed of two different polypeptide chains. The operon also contains several other genes (R, T, C, D, E, G, And Rib) that are thought to code for enzymes which produce the substrates for the light-emitting reaction. These substrates are called luciferins and are long chain fatty aldehydes. The Exercise Part I: Preparation of Competent Cells Review procedure on page 122 of your lab manual. This has been done for you prior to starting the experiment. Part II: Uptake of Plasmid by competent cells Part III: Selection on ampicillin plates Follow procedure in your lab manual. Note that to store your plates in the dark, you will put them in a paper sack and then in a box designated for your section Protocol Your cells must stay on ice at all times !!!! • Transfer 50 μL from the EC tube to each of the tubes labeled C (control), L (lux), and NP (no plasmid) • Incubate tubes for 20 min on ice • Incubate tubes for 30 sec at 42°C • Incubate on ice for 5 min • Add 500 μL of Nutrient Broth to each tube and incubate at 37°C for 30 min at 230 rpm • Spread 100 μL of each tube into an agar plate containing ampicillin (one plate per tube) (label plates) • Invert plates and incubate in dark Results Part IV: Examination of cultures in the dark You will have to attend open hours on: Section Open Hour Time (210 Noyes) A Thursday (11/12); 9 AM – 3 PM B Thursday (11/12); 11 AM – 5 PM C Thursday (11/12); 2 PM – 8 PM D Thursday (11/12); 2 PM – 8 PM E Thursday (11/12); 5 PM – 8 PM Friday (11/13); 9 AM – 11 AM F Friday (11/13); 11 AM – 3 PM G Friday (11/13); 11 AM – 3 PM H Friday (11/13); 11 AM – 3 PM