* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download pARA-R Restriction Digest: An Introduction to Plasmids and
Protein moonlighting wikipedia , lookup
Gene expression profiling wikipedia , lookup
Genome evolution wikipedia , lookup
Gene regulatory network wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Gene expression wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
List of types of proteins wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Non-coding DNA wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Molecular evolution wikipedia , lookup
Deoxyribozyme wikipedia , lookup
DNA vaccination wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Transformation (genetics) wikipedia , lookup
Genetic engineering wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Molecular cloning wikipedia , lookup
Genomic library wikipedia , lookup
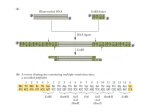
+ pARA-R Restriction Digest: An Introduction to Plasmids and Restriction Enzymes Laboratory 2a + Overview Purpose: Examine role of restriction endonucleses (enzymes) in genetic engineering Examine a bacterial plasmid and its use in biotechnology Methods: Preparing the pARA – R restriction digest + Introduction Restriction enzymes Cut DNA molecules from various organisms and recombine pieces Recombinant DNA Restrict the growth of viruses in bacteria Digest the DNA molecule at specific nucleotide sequences Restriction fragments DNA fragments Sticky ends Allow annealing and recombination of DNA fragments from different sources Engineering the Plasmid: ligation of rfp gene into p-ARA Bruce Wallace BamH I sticky end Hind III sticky end Hind III sticky end BamH I sticky end + Introduction Bacterial plasmids Circular pieces nonessential DNA found in bacteria Can be engineered to carry genes Express proteins encoded by these genes pARA – R Recombinant DNA plasmid Engineered to express rfp gene Produces a mutant Red Fluorescent Protein (mFP) Restriction digest of pARA-R Recombinant plasmid of interest pARA-R 4720 bp rfp 702bp + Recombinant Plasmid pARA - R Important control elements: araC Protein to help bacteria make other proteins Encoded by genes inserted into plasmid pBAD Site where RNA polymerase binds to initiate transcription rfp Hind III & BamH I Restriction enzymes ampr Antibiotic resistance gene Encodes beta lactamase + Materials Reagents: Equipment & Supplies pARA-R (70 ng / μL) P-20 micropipette and tips Restriction enzymes 1.5 mL microfuge tubes BamH I Minicentrifuge Hind III 37oC water bath Markers 2.5x restriction buffer Distilled water (dH2O) + What will you need to do? Aliquot: pARA – R Enzyme mix 2.5x restriction buffer Turn on water bath the day before lab 37oC + Methods + 1. Preparing the pARA-R Restriction Digest Three tubes: pARA-R Enzyme mix 2.5x restriction buffer Tube 2.5x buffer dH2O pARA - R Enzyme mix Total volume A+ 4 μL --------- 4 μL 2 μL 10 μL A- 4 μL 2 μL 4 μL -------- 10 μL Obtain 2 clean 1.5 mL microfuge tubes Label as “A+” and “A-” Use fresh tip and add 4 μL of 2.5x restriction buffer to both tubes Add 2 μL dH2O to “A-” Fresh tip and add 4 μL of pARA – R to both tubes + 1. Preparing the pARA-R Restriction Digest A+ tube Teacher will dispense enzyme mix 2 μL Cap tubes and gently “flick” to mix Tube 2.5x buffer dH2O pARA R Enzyme mix Total volume A+ 4 μL --------- 4 μL 2 μL 10 μL A- 4 μL 2 μL 4 μL -------- 10 μL Minifuge Balance with other tubes Place both tubes in 37oC water bath for 60 minutes Either go directly to Lab 4a or freeze until ready to complete + Conclusions Will result in 2 restriction fragments 4018 bp with ampr gene and control regions 702 bp with rfp gene Bruce Wallace Restriction analysis of pARA-R Restriction fragments after digest with Hind III and BamH I BamH I Hind III 4018 bp BamH I Hind III 702bp