* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Intracellular Compartments and Protein Sorting
Circular dichroism wikipedia , lookup
Protein domain wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein folding wikipedia , lookup
Homology modeling wikipedia , lookup
Protein structure prediction wikipedia , lookup
Trimeric autotransporter adhesin wikipedia , lookup
Protein moonlighting wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein purification wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
List of types of proteins wikipedia , lookup
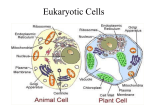
Organization Of Cell • Contains membraneenclosed organelles • Nucleus • Cytoplasm – Cytoplasmic organelles – Cytosol Transporting Proteins To Organelles • Synthesis begins in cytosol • Several mechanisms for transporting to organelles Sorting Signals • Segment(s) of amino acids direct protein to an organelle • Recognized by sorting receptors • Proteins with no sorting signal remain in cytosol Examples Of Signal Sequences • Sequence variability; physical properties often important Studying Signal Sequences • Functional signal sequences determined by experimental manipulation of proteins to alter their localization Nuclear Pore Complex • Multi-protein complex composed of nucleoporins • Diffusion of small molecules • Selective gate for proteins Nuclear Import & Export • Nuclear import receptor binds NLS of protein to be imported • Cargo-bound import receptor binds nucleoporins • Nuclear export is similar: export receptor binds to NES Functions Of Peroxisomes • Enzymes produce and consume H202 to oxidize organic substrates RH2 + O2 → R + H202 (various enzymes) H202 + R'H2 → R' + 2H20 (catalase) 2 H202 → 2H20 + O2 (catalase) • Synthesis of plasmalogens Import Into Peroxisomes • • • • Signal sequence often at C-terminus Some proteins with sequence near N-terminus Peroxins (receptors, docking proteins) participate in transport Inherited defects in peroxin genes such as Zellweger syndrome Transport Into Mitochondria • Have own genome for some proteins; maternally inherited • Nuclear genome encodes most proteins; synthesized in cytosol and imported Endoplasmic Reticulum • Site of synthesis for all proteins destined for secretion, the plasma membrane, lysosomes, endosomes, the Golgi, or the ER itself Docking Protein Onto ER Membrane • • • • Signal sequence contains hydrophobic amino acids SRP binds to signal sequence as it emerges from ribosome Co-translational transport onto ER membrane Start transfer through translocator as translation continues Soluble Protein Into ER Lumen • Signal sequence at N-terminus • Co-translational transport and translocation through membrane • Cleavage of signal sequence ER Transmembrane Protein With N-Terminal Signal • N-terminal sequence for transport and start transfer • Additional internal hydrophobic segment – Acts to stop transfer – Remains as membrane-spanning segment ER Transmembrane Protein With Internal Signal • Internal sequence for transport and start-transfer • Remains as membrane-spanning segment • Two orientations of signal sequence ER Multi-pass Transmembrane Protein • Multiple internal start and stop tranfer sequences N-Linked Glycosylation • Glycoproteins made in ER • Oligosaccharide precursor added to asparagine residues in ER • Processing in Golgi removes some sugar residues Glycosylation In ER • Transfer of preformed oligosaccharide precursor • Catalyzed by oligosaccharyl transferase • Oligosaccharide to be transferred attached to dolichol Synthesis Of Dolichol-linked Oligosaccharide • Stepwise addition of sugar resides • Nucleotide-sugar intermediates donate sugars • Monosaccharide-linked dolichol molecules transfer sugars O-Linked Glycosylation • Oligosaccharide linked to hydroxyl groups of serine, threonine, or hydroxylysine residues • Occurs in Golgi Protein Folding In ER • Chaperones aid in folding • Improperly folded proteins enter cytosol through translocator; deglycosylated, ubquitylated, and degraded Addition Of GPI Anchor • Some proteins destined for plasma membrane • Hydrophobic C-terminal sequence • C-terminus cut and preassembled GPI attached Vesicular Transport • Vesicle buds off from one compartment and fuses with another • Compartments that communicate are topologically equivalent Protein Coats In Vesicular Transport • Cage of proteins covering cytosolic surface • Concentrates membrane proteins and deforms membrane Clathrin Structure • Subunits associate into triskelion • Convex framework of triskelions on cytosolic surface Formation Of Clathrin-coated Vesicle Clathrin coat: • introduces curvature leading to formation of bud • linked to transmembrane cargo receptors by adaptins • removed after transport vesicle is pinched off Organization Of Golgi Apparatus CGN Golgi Stack cis cisterna medial cisterna trans cisterna TGN ER → CGN → cis-, medial-, trans cisternae → TGN Transporting From ER To CGN • Exit signal on soluble cargo interacts with transmembrane receptor • Exit signal on receptor interacts with protein coat ER Resident Proteins Golgi → ER • Sorting signal for retrieval of ER proteins that enter Golgi Membrane proteins: KKXX- (COO-) Soluble proteins: KDEL- (COO-) • Transmembrane receptor for KDEL that binds coat proteins Processing N-linked Oligosaccharides • Two classes formed by modifications to precursor in Golgi Complex oligosaccharides High-mannose oligosaccharides Lysosomes • Controlled digestion of macromolecules Sorting By Recognizing M6P • M6P added to lysosomal hydrolases in CGN • Transmembrane M6P receptors in TGN interact with coat proteins Specific Addition Of M6P • Signal patch recognized by GlcNAc phosphotransferase Lysosomal Storage Diseases • Genetic defects affecting lysosomal hydrolases • Accumulation of undigested material in lysosomes • Tay-Sachs disease – defective hexosaminidase A gene – accumulation of ganglioside GM2 • Gaucher disease – defective glucocerebrosidase gene • Hurler’s disease – defective a-L-iduronase gene • I-cell disease – most hydrolases missing from lysosomes – inclusion bodies – defective GlcNAc phosphotransferase gene Protein Sorting In TGN • Lysosomes • Constitutive secretory pathway – Transport vesicles from TGN to plasma membrane – Default pathway • Regulated secretory pathway – Sorting signal targets to special secretory vesicles • Pathways initially involve ER signal sequence, SRP Exocytosis • Constitutive secretory pathway: transport continually from TGN to plasma membrane • Regulated secretory pathway: store in secretory vesicles until stimulated Endocytosis • Material to be ingested becomes enclosed by plasma membrane as it invaginates • Buds off to form endocytic vesicles Endocytic/Degradation Pathways Delivering materials to lysosomes for digestion: • Endocytosis – Pinocytosis – Receptor-mediated endocytosis – Phagocytosis • Autophagy Receptor-mediated Endocytosis Of LDL • Cholesterol molecules in LDL organized by protein that binds to LDL receptor • LDL receptor interacts with clathrin-coated pit • Mutation in LDL receptor causes familial hypercholesterolemia Sorting In Early Endosome • Endocytoic vesicles fuse with early endosomes • Ligand-receptor dissociation • Possible fates of receptor: recycling, transcytosis, degradation Endocytic Pathway Of LDL • LDL receptor recycled to plasma membrane • LDL degraded in lysosome to release free cholesterol From Early Endosomes To Lysosomes • Early endosomes form multivesicular bodies by enclosing invaginations • Turn into late endosomes that are more acidic • Form lysosomes by receiving hydrolases, further acidification