* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download (TH) and Pulmonary Hypoplasia with Anasarca
Genetic testing wikipedia , lookup
DNA supercoil wikipedia , lookup
Public health genomics wikipedia , lookup
Epigenomics wikipedia , lookup
Gene expression programming wikipedia , lookup
Non-coding DNA wikipedia , lookup
X-inactivation wikipedia , lookup
Genome (book) wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Genetic engineering wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Koinophilia wikipedia , lookup
Microsatellite wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Population genetics wikipedia , lookup
DNA paternity testing wikipedia , lookup
Frameshift mutation wikipedia , lookup
Helitron (biology) wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
History of genetic engineering wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Saethre–Chotzen syndrome wikipedia , lookup
Designer baby wikipedia , lookup
Genealogical DNA test wikipedia , lookup
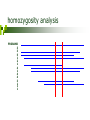
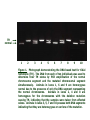
Tibial Hemimelia (TH) and Pulmonary Hypoplasia with Anasarca (PHA) _____________________ What are they, where are they and how are they relevant Jonathan Beever, PhD University of Illinois November 2, 2006 tibial hemimelia (th) skeletal defects other defects failure of pelvic fusion – abdominal hernia shortened or absent tibia – severe distortion of rear leg structure failure of proper neural tube closure – exposure of brain or spinal tissue cryptorchidism, failed Mullerian duct development invariably lethal calves may be live born – fail to thrive, euthanized background recognized in Galloway cattle in early 70’s (Ojo et al. 1974) documented sire test/selection program in UK genetic inheritance Reported in in Shorthorn cattle in 2000 (Lapointe et al. 2000) 3 of 6 calves reported of Canadian origin ancestry common among all calves genetics unaffected parents (i.e., normal is dominant) equal frequency among sexes pedigree analysis reveals common ancestry on both sides of pedigree expected ratios of offspring among matings between carrier (heterozygous) parents 3:1 ratio of normal to affected offspring recessive Mendelian inheritance animals homozygous for defect (mutation) are affected both parents of affected calves must be carriers potential impact worldwide putative common ancestor is early Irish import one of few direct imports – extensive use circa ~1975 – multiple generations of dispersion multiplied in US – exportation of germplasm US (2004 perspective) more than half of the top 10 sires for number of Shorthorn registrations are putative carriers popular club calf sire is suspected carrier estimated 80,000 units of semen sold In 2005, 21 of 24 black composite AI sires offered by a single vendor are tested as carriers how to find the defective gene identification of appropriate pedigree/population material collect DNA samples ~60 individuals of known genotype status within “nuclear” families genetic marker screening even distribution/coverage across genome panel of 263 markers prioritize chromosomes for analysis comparative biology/genomics homozygosity analysis PROBAND 3 3 5 1 2 1 4 1 4 4 3 1 1 1 comparative genomics mutation screening complete DNA sequencing of causative gene ~140,000 base pairs resequencing of animals of known genotype normal, carrier and affected no variation in DNA sequence that was consistent between all known animals inability to resequence portion of gene in affected calves significant portion (30%) of gene absent in affected calves TH normal 1 2 3 4 5 6 7 8 9 10 Figure 1. Photograph demonstrating the DNA-based test for tibial hemimelia (TH). The DNA from each of ten individuals was used to determine their TH status by PCR amplification of the normal chromosome segment and the mutated chromosomal segment simultaneously. Animals in lanes 1, 6 and 9 are homozygous normal due to the presence of only the DNA segment representing the normal chromosome. Animals in lanes 2, 4 and 8 are homozygous for the chromosome with the deletion mutation causing TH, indicating that the samples were taken from affected calves. Animals in lanes 3, 5, 7 and 10 possess both DNA segments indicating that they are heterozygous or carriers of the mutation. validation blind testing of 45 animals of known status 100% random testing of ~300 phenotypically normal individuals none accurate homozygous for mutation testing of 7 known sires confirmed by ASA genetic defect policy only 6 of 7 genotype as carriers resolution different/inconsistent phenotype? Pulmonary Hypoplasia with Anasarca (PHA) all affected calves from inconsistent sire genotype as homozygotes for identified mutation all affected calves parentally verify to sire except for DNA markers adjacent to causative gene 2nd mutation – complete deletion of gene complete deletion of 4 genes (460,000 bp) very rare frequency as compared to first curiosities selection paradox carriers are the “best” is there a quantitative measure to define best? non-pathological heterozygotes? manifestation in structural differences in hindquarters remember gene function perstistance and selective increase in the breeding population over time almost impossible to “dilute” pulmonary hypoplasia with anasarca (PHA) pulmonary hypoplasia anasarca tremendous fluid accumulation in affected calves lack of lymphatic development absence of lymph duct and nodes, athymia invariably lethal absent or near absence of lungs normal cardiovascular system all near term calves born dead other early embryonic lethal – increased open rate after confirmed pregnancy genetics unaffected parents (i.e., normal is dominant) equal frequency among sexes pedigree analysis reveals common ancestry on both sides of pedigree deficiency of affected calves given suspected frequency recessive Mendelian inheritance affected pedigrees in both Shorthorn and Maine Anjou breeds potential impact putative common ancestor is early French or Canadian import circa ~1975 – multiple generations of dispersion multiplied in US 40 of 121 popular club calf sires are carriers potential carriers for phenotypic selection in the >80% of sons in AI service that are sired by a popular carrier club calf sire are carriers mutation screening complete DNA sequencing of causative gene resequencing of animals of known genotype normal, carrier and affected single missense mutation common to modern Shorthorn, Maine Anjou and composite cattle validation “blind” testing of 144 animals of known status 100% random testing of ~1000 phenotypically normal individuals none accurate homozygous for mutation 4 suspect sires test normal insufficient evidence of their status risk assessment do you care? methods to assess risk pedigree analysis do your pedigrees contain suspect individuals? including “modern” sires that have been tested diagnostic screening random testing within your herd suspect pedigree representation pedigree assessment at what point in a pedigree doesn’t it matter anymore? how many generations? n (1/2) – probability of carrier n = number of generations between known carrier and individual in question 1 generation = 50% 3 generations = 12.5% 8 generations = 0.4% additive – consider all suspect individuals with independent paths to individual breeding management education is key understand the possibilities – desired outcome do nothing vs. “kill ‘em all” up to individual breeders vs. mandatory testing and culling of all carrier animals accurate identification of carriers selective vs. comprehensive testing programs voluntary vs. mandatory what to test expense vs. outcome low cost – no affected calves born sires only – no affected calves born to TH-Free sires moderate sires, herd matriarchs and annual replacement heifers highest cost – on the road to elimination cost – complete management all animals in the herd does not imply elimination, only management acknowledgements Charles P. Hannon, DVM Nick Steinke Brandy Marron Geri Thurneau USDA CSREES/ARS – LGSI