* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download biochemistry-micromolecules
Bottromycin wikipedia , lookup
Polyadenylation wikipedia , lookup
RNA silencing wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Epitranscriptome wikipedia , lookup
Gene expression wikipedia , lookup
Citric acid cycle wikipedia , lookup
Non-coding RNA wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Expanded genetic code wikipedia , lookup
Genetic code wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Proteolysis wikipedia , lookup
Metalloprotein wikipedia , lookup
List of types of proteins wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Deoxyribozyme wikipedia , lookup
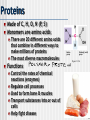
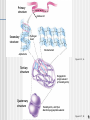
Organic vs. Inorganic All compounds can be separated into two groups: Inorganic • Doesn’t contain carbon • Non-living • Examples: Oxygen gas, metals, rocks, water Organic • Contains carbon • Living (or dead) • Examples: wood, grass, diamonds, petroleum 1 Polymerization Monomers One unit of a compound Polymers Many monomers combine to make a polymer Macromolecules Many large molecules combined 2 Carbohydrates Made of C, H, O Functions Main energy source in organisms Structural component in plants (CELLULOSE) Types Sugars • gives off energy when broken down • Ex. Sucrose, fructose, glucose Starches • used as a storage molecule for sugars • Ex. Bread, rice, pasta, corn 3 Lipids Made of C, H, O Commonly called fats, oils, waxes Functions in the form of glycerol and fatty acid chains Storage of energy Parts of biological membranes Water proof coverings Chemical messengers (steroids) Insoluble in water Ex. Lard, butter, oil, hormones, steroids 4 Fatty acid Figure 3.8B Figure 3.8C Saturated They fats (lard) lack double bonds are solid at room temperature 5 Nucleic acids Made of C, H, O, N, P Monomers are called nucleotides Functions Nucleotides are made up of a 5-carbon sugar, phosphate group and a nitrogen base Store hereditary information Transmit hereditary information Nitrogenous base (A) Phosphate group Sugar Figure 3.20A Two types RNA (ribonucleic acid) DNA (deoxyribonucleic acid) 6 RNA vs DNA There are THREE main differences between DNA & RNA The sugar • In DNA its DEOXYribose sugar • In RNA it’s Ribose sugar Number of strands • DNA is usually double stranded • RNA is ONLY single stranded Nitrogen Bases • DNA • Adenine pairs with Thymine • Guanine pairs with Cytosine • RNA • Adenine pairs with Uracil • Guanine pairs with Cytosine 7 Proteins Made of C, H, O, N (P, S) Monomers are amino acids There are 20 different amino acids that combine in different ways to make millions of proteins The most diverse macromolecules Functions Control the rates of chemical reactions (enzymes) Regulate cell processes Used to form bone & muscles Transport substances into or out of cells Help fight disease Amino group Carboxyl (acid) group Figure 3.12A 8 Primary structure Amino acid Hydrogen bond Secondary structure Pleated sheet Alpha helix Figure 3.15, 16 Tertiary structure Polypeptide (single subunit of transthyretin) Quaternary structure Transthyretin, with four identical polypeptide subunits Figure 3.17, 18 9 Enzymes Special PROTEINS Act as biological CATALYSTS: speed up the rate of a chemical reaction by lowering the activation energy of the reaction Activation Energy: energy needed to transform reactant substances into product substances Reaction pathway without enzyme Reactants Reaction pathway with enzyme Activation energy without enzyme Activation energy with enzyme Products 10 • Enzymes are specific in the reactions they catalyze (Lock and Key model) • They will only catalyze one specific substance, in one direction (a -> b, but not b -> a) • They are reusable • A substance that an enzyme reacts on is called the enzyme’s substrate • Only the active site in the enzyme actually binds to the substrate • Enzymes end in –ase • Example: amylase, helicase 11 Factors Affecting Enzyme Activity: • PH • Temperature • Salt concentration • Enzymes lose their shape easily (denature) • Shape is very important in enzyme activity! 12