* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download lec 7 Metabolism of purine nucleotides
Artificial gene synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Peptide synthesis wikipedia , lookup
Paracrine signalling wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Metalloprotein wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Biochemical cascade wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Biochemistry wikipedia , lookup
Butyric acid wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Citric acid cycle wikipedia , lookup
Amino acid synthesis wikipedia , lookup
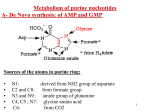
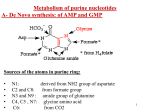
Metabolism of purine nucleotides 1- Biosynthesis of purine nucleoetides A- De novo biosynthesis (10 % in liver mainly) B- Salvage pathway (90 % in other tissues) 2- Degradation of purine nucleotides 3- Disorders of purine nucleotides A- Gout B- Lesch Nyhan syndrome Remember that purine nucleotides are: AMP, ADP , ATP: adenosine mono (or di or tri) phosphate GMP, GDP, GTP: Guanosine mono (or di or tri) phosphate 2 A- De Novo synthesis of AMP and GMP Site: Mainly in the liver (in the cytoplasm) Sources of the atoms in purine ring: • • • • • N1: derived from NH2 group of aspartate C2 and C8: from formate group N3 and N9 : amide group of glutamine C4, C5 , N7: glycine amino acid C6: from CO2 3 3 4 Notes on de novo purine nucleotides biosynthesis: 1. Phosphoribosyl pyrophosphate (PRPP) is the source of ribose 5-phosphate. PRPP is the active form of ribose-5-phosphate 2. Pyrophosphate is removed from PRPP and substituted with NH2 group of glutamine to form phosphoribosylamine. This step is the rate limiting step and catalyzed by amidophosphoribosyl transferase 5 The rate limiting step in de novo biosynthesis 6 3. The ring is then formed from their atoms sources (aspartic, glutamine, glycine, etc). 4. The pathway ends with the formation of a purine nucleotide called : Inosine monophosphate (IMP) which is the precursor of AMP and GMP which then converted into ATP and GTP, respectively AMP and GMP ← 7 NB: IMP is a nucleotide contain purine base which is hypoxanthine (6 –oxy purine). Hypoxanthine is a purine base not enter in DNA or RNA structure hypoxanthine 8 8 IMP is converted into AMP by the addition of aspartate. NH2 group of aspartate replace oxy group in carbon 6 to form AMP. IMP is converted into GMP through the addition of glutamine. NH2 group of glutamine is added to carbon 2 to form GMP. 6 2 9 Conversion of IMP to AMP and GMP (for illustration) 10 Regulation of the pathway: amidophosphoribosyl transferase catalyses the rate limiting step of the pathway. Activator of the amidotransferase: This enzyme is activated by PRPP. So PRPP is an activator of the pathway. Increased PRPP leads to overproduction of purine nucleotides. Inhibitors of the amidotransferase: The enzyme is inhibited by the final products of the pathway (IMP, AMP and GMP). 11 B- Salvage pathway of purines: or resynthesis of purine nucleotides: De novo biosynthesis occur in liver due to presence of enzymes. Other tissues can’t do de novo synthesis. In these organs, free purine bases (guanine, hypoxanthine and adenine) reacts with PRPP again to resynthesize purine nucleotides. These free purine bases are obtained from diet or result during breakdown of purine nucleotides (see purine catabolism) 12 Salvage pathway needs two enzymes: 1- Adenine phosphoribosyl transferase (APRTase) 2- Hypoxanthine-guanine phosphoribosyl transferase (HGPRTase) Both enzymes use PRPP as the source of ribose-5-phosphate The first enzyme catalyze the transfer of ribose-5-P from PRPP to adenine to synthesize AMP. → APRTase Adenine + PRPP AMP 13 The second salvage enzyme (HGPRTase) catalyzes the transfer of ribose-5-P into hypoxanthine or guanine to regenerate IMP or GMP. → HGPRTase Hypoxanthine + PRPP → IMP HGPRTase Guanine + PRPP GMP 14 Catabolism (breakdown) of purine nucleotides Uric acid is the end product of purine metabolism in human. AMP or GMP is metabolized to give hypoxanthine which is then converted into xanthine and finally into uric acid as in the next slide. Most of uric acid is excreted by the kidney. The remaining uric acid travels through the intestines, where bacteria help break it down. Normally these actions keep the level of uric acid in the blood plasma at a healthy level, which is below 6.8 mg/dL. But under certain circumstances, the body produces too much uric acid or removes too little. In either case, concentrations of uric acid increase in the blood. This condition is known as hyperuricemia. 15 16 Disorders of purine nucleotides metabolism A- Gout: is a disorder characterized by high levels of uric acid in blood (hyperuricemia), with deposition of monosodium urate crystals in special sites in the body like joints, and surrounding tissues and sometimes in the kidney. Gout is a type of arthritis Urate crystals are detected in synovial fluid of the joint 17 Causes: Primary causes such as 1- decreased excretion of uric acid by the kidney due to renal disease, Sometimes it is inherent. 2-defect in purine metabolism: increased synthesis of purine nucleotides which may be idiopathic (with unknown cause) or due to increased levels of PRPP that stimulate synthetic pathway of purine nucleotides. The increased purine lead to increased uric acid production Secondary causes: such as diet rich in purines such as red meat, duck, liver, xanthine beverages like tea, coffee, cola or due to medication Symptoms: 1- Hyperuricemia: increased uric acid levels in blood 2- arthritis, inflammation especially in joints due to deposition of urate crystals leading to hot red and swollen joints with severe pain.18 3- redness, swelling of big toe. 4- it may also present as tophi (masses of urate crystals deposited under skin) appears after several years. 5- It may lead to kidney stones, Tophi, in chronic cases, lumpy deposits of urate just under the skin 19 Treatment: Allopurinol, analogue of hypoxanthine (structurally similar). It competitively inhibits xanthine oxidase, so prevents the conversion of hypoxanthine to xanthine and xanthine to uric acid. Uricosuric agents: drugs used to increase excretion of uric acid by the kidney such as probenecid. 20 Anti-inflammatory drugs is recommended also. 21 B-Lesch –Nyhan syndrome: Cause: inherited disease resulting from complete deficiency (absesnce) of HGPRTase → block (inhibit) salvage pathway of guanine and hypoxanthine → ↓ use of PRPP in salvage pathway and ↑ its use in purine synthesis leading to overproduction of purine nucleotides which by catabolism, will give increased levels of uric acid Symptoms: appear at age 3-6 months. The first symptom is orange colored crystals in the diaper of the baby. 1- Hyperuricemia: in aggressive way than in gout. 2- urate kidney stones: Some symptoms of unknown mechanism are: 3- mental retardation 4- involuntary movements of legs and arms 5- lack of muscle coordination 6- self mutilation (biting of fingers and lips 22 22 leading to lip lesions).