* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pathways that Harvest and Store Chemical Energy
Survey
Document related concepts
Fatty acid metabolism wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Electron transport chain wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Citric acid cycle wikipedia , lookup
Transcript
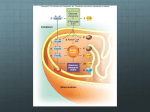
6 Pathways that Harvest and Store Chemical Energy Energy flow and chemical recycling in ecosystems Concept 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism Five principles governing metabolic pathways: 1. Chemical transformations occur in a series of intermediate reactions that form a metabolic pathway. 2. Each reaction is catalyzed by a specific enzyme. 3. Most metabolic pathways are similar in all organisms. Concept 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism 4. In eukaryotes, many metabolic pathways occur inside specific organelles. 5. Each metabolic pathway is controlled by enzymes that can be inhibited or activated. Figure 6.1 The Concept of Coupling Reactions Figure 9.2 A review of how ATP drives cellular work Figure 6.2 ATP Substrate-level phosphorylation Concept 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism Energy can also be transferred by the transfer of electrons in oxidation–reduction, or redox reactions. • Reduction is the gain of one or more electrons. • Oxidation is the loss of one or more electrons. Concept 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism Oxidation and reduction always occur together. Methane combustion as an energy-yielding redox reaction NAD+ as an electron shuttle Concept 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism Reduction of NAD+ is highly endergonic: NAD H 2e NADH Oxidation of NADH is highly exergonic: NADH H 1 2 O2 NAD H 2O Concept 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism Oxidative phosphorylation couples oxidation of NADH: NADH NAD H 2e energy with production of ATP: energy ADP Pi ATP Figure 6.5 A Chemiosmosis Figure 6.5 B Chemiosmosis http://www.stolaf.edu/people/giannini/flas hanimat/metabolism/atpsyn1.swf http://www.stolaf.edu/people/giannini/flash animat/metabolism/atpsyn2.swf Figure 6.8 Energy Metabolism Occurs in Small Steps Figure 6.9 Energy-Releasing Metabolic Pathways An overview of cellular respiration Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy Glycolysis: ten reactions. Takes place in the cytosol. Final products: 2 molecules of pyruvate (pyruvic acid) 2 molecules of ATP 2 molecules of NADH Figure 6.10 Glycolysis Converts Glucose into Pyruvate (Part 1) Figure 6.10 Glycolysis Converts Glucose into Pyruvate (Part 2) Figure 6.10 Glycolysis Converts Glucose into Pyruvate (Part 3) Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy Examples of reaction types common in metabolic pathways: Step 6: Oxidation–reduction Step 7: Substrate-level phosphorylation text art pg 107 here Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy Pyruvate Oxidation: Products: CO2 and acetate; acetate is then bound to coenzyme A (CoA) Figure 6.11 The Citric Acid Cycle Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy Final reaction of citric acid cycle: Figure 6.12 Electron Transport and ATP Synthesis in Mitochondria http://www.stolaf.edu/people/giannini/flashanimat/metabolism/mido%20e%20t ransport.swf Concept 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy About 32 molecules of ATP are produced for each fully oxidized glucose. The role of O2: most of the ATP produced is formed by oxidative phosphorylation, which is due to the reoxidation of NADH. Concept 6.3 Carbohydrate Catabolism in the Absence of Oxygen Releases a Small Amount of Energy Under anaerobic conditions, NADH is reoxidized by fermentation. There are many different types of fermentation, but all operate to regenerate NAD+. The overall yield of ATP is only two—the ATP made in glycolysis. Concept 6.3 Carbohydrate Catabolism in the Absence of Oxygen Releases a Small Amount of Energy Lactic acid fermentation: End product is lactic acid (lactate). NADH is used to reduce pyruvate to lactic acid, thus regenerating NAD+. Figure 6.13 A Fermentation Concept 6.3 Carbohydrate Catabolism in the Absence of Oxygen Releases a Small Amount of Energy Alcoholic fermentation: End product is ethyl alcohol (ethanol). Pyruvate is converted to acetaldehyde, and CO2 is released. NADH is used to reduce acetaldehyde to ethanol, regenerating NAD+ for glycolysis. Figure 6.13 B Fermentation Figure 6.14 Relationships among the Major Metabolic Pathways of the Cell Concept 6.4 Catabolic and Anabolic Pathways Are Integrated Catabolism: Polysaccharides are hydrolyzed to glucose, which enter glycolysis. Lipids break down to fatty acids and glycerol. Fatty acids can be converted to acetyl CoA. Proteins are hydrolyzed to amino acids that can feed into glycolysis or the citric acid cycle. How DO cells break down a triglyceride to produce ATP? b-oxidation! http://higheredbcs.wiley.com/legacy/college/pratt/0471393878/st udent/animations/fatty_acid/index.html Concept 6.4 Catabolic and Anabolic Pathways Are Integrated Anabolism: Many catabolic pathways can operate in reverse. Gluconeogenesis—citric acid cycle and glycolysis intermediates can be reduced to form glucose. Acetyl CoA can be used to form fatty acids. Some citric acid intermediates can form nucleic acids. The control of cellular respiration Concept 6.5 During Photosynthesis, Light Energy Is Converted to Chemical Energy Photosynthesis involves two pathways: Light reactions convert light energy into chemical energy (in ATP and the reduced electron carrier NADPH). Carbon-fixation reactions use the ATP and NADPH, along with CO2, to produce carbohydrates. Figure 6.15 An Overview of Photosynthesis Concept 6.5 During Photosynthesis, Light Energy Is Converted to Chemical Energy Light is a form of electromagnetic radiation, which travels as a wave but also behaves as particles (photons). Photons can be absorbed by a molecule, adding energy to the molecule—it moves to an excited state. Figure 6.16 The Electromagnetic Spectrum Figure 10.6 Why leaves are green: interaction of light with chloroplasts Concept 6.5 During Photosynthesis, Light Energy Is Converted to Chemical Energy Pigments: molecules that absorb wavelengths in the visible spectrum. Chlorophyll absorbs blue and red light; the remaining light is mostly green. Absorption spectrum—plot of light energy absorbed against wavelength. Action spectrum—plot of the biological activity of an organism against the wavelengths to which it is exposed Determining an absorption spectrum Figure 6.17 Absorption and Action Spectra Evidence that chloroplast pigments participate in photosynthesis: absorption and action spectra for photosynthesis in an alga Figure 6.18 The Molecular Structure of Chlorophyll (Part 1) Figure 6.18 The Molecular Structure of Chlorophyll (Part 2) Excitation of isolated chlorophyll by light Figure 6.19 Noncyclic Electron Transport Uses Two Photosystems A mechanical analogy for the light reactions Concept 6.5 During Photosynthesis, Light Energy Is Converted to Chemical Energy ATP is needed for carbon-fixation pathways. Cyclic electron transport uses only photosystem I and produces ATP; an electron is passed from an excited chlorophyll and recycles back to the same chlorophyll. Cyclic electron flow The light reactions and chemiosmosis: the organization of the thylakoid membrane Figure 6.21 The Calvin Cycle Concept 6.6 Photosynthetic Organisms Use Chemical Energy to Convert CO2 to Carbohydrates 1. Fixation of CO2: CO2 is added to ribulose 1,5-bisphosphate (RuBP). Ribulose bisphosphate carboxylase/oxygenase (rubisco) catalyzes the reaction. A 6-carbon molecule results, which quickly breaks into two 3-carbon molecules: 3phosphoglycerate (3PG). Figure 6.22 RuBP Is the Carbon Dioxide Acceptor Concept 6.6 Photosynthetic Organisms Use Chemical Energy to Convert CO2 to Carbohydrates 2. 3PG is reduced to form glyceraldehyde 3phosphate (G3P). C4 leaf anatomy and the C4 pathway C4 and CAM photosynthesis compared A review of photosynthesis