* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Carbon Compounds
Fatty acid metabolism wikipedia , lookup
Plant nutrition wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Biosynthesis wikipedia , lookup
Isotopic labeling wikipedia , lookup
Carbon sink wikipedia , lookup
Photosynthesis wikipedia , lookup
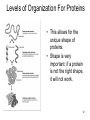
Proteolysis wikipedia , lookup
Metalloprotein wikipedia , lookup
Microbial metabolism wikipedia , lookup
Carbon Compounds 1 Organic Compound? • In Biology, the word organic means “relating to organisms.” • All organic compounds contain covalently bound carbon. • Organic compounds can also be synthesized in the lab. 2 The Chemistry of Carbon • Carbon atoms have four valence (outer shell) electrons, allowing carbon to form covalent bonds with many elements including hydrogen, oxygen, phosphorus, sulfur and nitrogen. 3 The Chemistry of Carbon Cont. • Carbon can also bond to other carbon atoms, which gives carbon the ability to form chains that are almost unlimited in length. • These carbon-carbon bonds can be single, double, or triple. • The chains may be straight, branched, or even ring-shaped. • Therefore, carbon is unique in that it can form millions of different large and complex structures. 4 Macromolecules • They are giant molecules which are made from many smaller molecules. • They are formed by a process known as polymerization, in which large compounds are built by joining smaller ones together. – The smaller units, or monomers, join to form polymers 5 Carbohydrates - Sugars • Main source of energy for organisms • Can also be used for structural purposes • Made of C, H, and O; usually in a 1:2:1 ratio • The monomers of carbohydrates are called monosaccharides, like glucose, fructose (in fruits) and galactose (in milk). • The breakdown of monosaccharides supplies immediate energy. • Usually end in –ose. • Extra sugar is stored as macromoleucles called polysaccharides. • Polysaccharides are made from monosaccharides. 6 How Polysaccharides Are Used • Many animals store extra sugar as glycogen. – Glycogen stored in your muscle supplies energy for contractions. – Glycogen stored in your liver is released when glucose in your blood runs low. • Plants store excess sugar as starch. • Plants also make cellulose, a strong, rigid fiber used for support. 7 Lipids • Ex: fats, oils, waxes • Uses: long-term energy storage, insulation, waterproof covering, part of biological membranes, chemical messengers (steroids) • contain mostly carbon and hydrogen • Many lipids are formed when a glycerol molecule combines with a fatty acid. – If all carbon atoms have only single bonds, the lipid is saturated. – If there is at least one double bond between carbon atoms, the lipid is unsaturated. – Unsaturated lipids like cooking oils tend to be liquid at room temperature. 8 Nucleic Acids • Store and transmit hereditary, or genetic, information • Contain hydrogen, carbon, nitrogen, oxygen, and phosphorus • Nucleic acids are polymers assembled from individual monomers known as nucleotides. • Nucleotides consist of three parts: a 5-carbon sugar, a phosphate group, and a nitrogen base. • Examples are DNA and RNA 9 Proteins • Contain nitrogen, carbon, hydrogen, and oxygen. • The monomers of proteins are amino acids. • Proteins provide structural support in bones and muscles. • They form parts of cell membranes and function as hormones to regulate the body. • They form antibodies to protect against infection. • Some proteins also control the rate of chemical reactions. 10 Proteins Cont. • When the amino acids join, they form a polymer called a polypeptide. The monomers are held together by peptide bonds. • More than 20 different amino acids are found in nature. • Since the R-group varies, it allows for much variety. That is why proteins have so many functions. 11 Levels of Organization For Proteins • This allows for the unique shape of proteins. • Shape is very important; if a protein is not the right shape, it will not work. 12