* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Photosynthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Biosynthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biochemical cascade wikipedia , lookup
Basal metabolic rate wikipedia , lookup
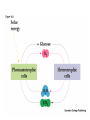
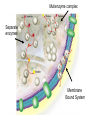
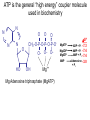
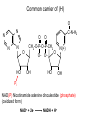
Lecture 19 – Quiz next Friday (Oct. 28) on glycolysis. – Metabolism and thermodynamics – (This material forward not on exam) Summary: various methods to increase rate • Increase frequency of the correct group in the correct place e.g. proximity effect • Lower EA by specific catalysis -acid-base catalysis, nucleophile or electrophile • Raise energy of reactants (closer to EA) - ring distortion, transition state analog • Provide alternate low EA pathway - covalent catalysis. • Michaelis Menten vs. Allosterism • Lineweavear Burk • Eadie Hofstee • Competitive inhibition • Noncompetitive inhibition • Uncompetitive inhibition Terms to review for enzymes • • • • • • • • • Cofactor Coenzyme Prosthetic group Holoenzyme Apoenzyme Lock and Key Transition analog model Induced fit Active site, binding site, recognition site, catalytic site To be included on the quiz Introduction to metabolism: Main Functions • To obtain energy for growth • From the degradation of energy rich compounds (chemotrophy) • • • • • Organotrophs-organism obtains H or e- from organic compounds Lithotrophs-uses an inorganic substrate to obtain reducing equivalents. From light (photosynthesis) Phototrophs To use energy to assemble precursors into building blocks of the cell and to convert those building blocks into proteins, carbohydrates, lipids, etc. • Autotrophs (starting carbon source is CO2) • Heterotroph (use preformed complex organics) To degrade and recycle unnecessary metabolites or compounds no longer in use by the cell. Metabolism • The sum of the chemical changes that convert nutrients into energy and the chemically complex products of cells • Hundreds of enzyme reactions organized into discrete pathways • Substrates are transformed to products via many specific intermediates • Metabolic maps portray the reactions • Intermediary metabolism A Common Set of Pathways • Organisms show a marked similarity in their major metabolic pathways • Evidence that all life descended from a common ancestral form • There is also significant diversity The Sun is Energy for Life • Phototrophs use light to drive synthesis of organic molecules • Heterotrophs use these as building blocks • CO2, O2, and H2O are recycled Metabolism • Metabolism consists of catabolism and anabolism • Catabolism: degradative pathways – Usually energy-yielding! • Anabolism: biosynthetic pathways – energy-requiring! Metabolism is divided into 2 types A. Catabolism - degradative metabolism to yield CO2, simple metabolites, and energy. Accompanied by the release of G stored in complex molecules - stored as ATP, NADPH (generate free energy) B. Anabolism - Biosynthesis. Energy requiring step-uses building blocks, ATP and NADH/NADPH to form complex molecules. I. Primary Metabolism - metabolism used for the construction of essential building blocks and energy metabolism. Secondary Metabolisms - Other nonessential metabolism. “Chemical reactions that create diverse byproducts often unique to a taxon and generally not essential for survival and have no known metabolic role.” II. Catabolism and Anabolism • Catabolic pathways converge to a few end products • Anabolic pathways diverge to synthesize many biomolecules • Some pathways serve both in catabolism and anabolism • Such pathways are amphibolic Organization in Pathways • • • • Pathways consist of sequential steps The enzymes may be separate Or may form a multienzyme complex Or may be a membrane-bound system • New research indicates that multienzyme complexes are more common than once thought Mutienzyme complex Separate enzymes Membrane Bound System Organization of Pathways Closed Loop (intermediates recycled) Linear (product of rxns are substrates for subsequent rxns) Spiral (same set of enzymes used repeatedly) 5 principal characteristics of metabolic pathways 1. Metabolic pathways are irreversible. (large negative free energy change) 2. Catabolic and anabolic pathways must differ. 3. Every metabolic pathway has a first committed step. 4. All metabolic pathways are regulated. 5. Metabolic pathways in eukaryotic cells occur in specific cellular locations. Metabolism Proceeds in Discrete Steps •Enzyme specificity defines biosynthetic route •Controls energy input and output •Allow for the establishment of control points. •Allows for interaction between pathways Regulation of Metabolic Pathways • Pathways are regulated to allow the organism to respond to changing conditions. • Most regulatory response occur in millisecond time frames. • Most metabolic pathways are irreversible under physiological conditions. • Regulation ensures unidirectional nature of pathways. • Flow of material thru a pathway is referred to as flux. • Flux is regulated by supply of substrates, removal of products, and activity of enzymes Enzyme Regulation of Flux Common mechanisms • Feedback inhibition – product of pathway down regulates activity of early step in pathway • Feedforward activation – metabolite produced early in pathway activates down stream enzyme Metabolic Control Theory • Pathway flux is regulated by multiple enzymes in a pathway. • Control coefficient determined for each enzyme. = activity / enzyme concentration. • Enzymes with large control coefficients impt to overall regulation. • Recent finding suggest that the control of most pathways is shared by multiple pathway enzymes Regulating Related Catabolic and Anabolic Pathways • Anabolic & catabolic pathways involving the same compounds are not the same • Some steps may be common to both • Others must be different - to ensure that each pathway is spontaneous • This also allows regulation mechanisms to turn one pathway and the other off Gibb’s Free Energy • Useful to know how likely a reaction will occur. The measurement of driving force for all reactions is the decrease in G. G = H -TS Spontaneous reactions all have -G, but imply nothing about the rate! (G = 0 at equilibrium) This is related to the G of the entire system. For our purposes, dependent upon Gf of formation for the reactants and products. Gibb’s Free Energy Oxaloacetate (OAA) + H+ CO2 (g) + pyruvate G for the reaction = Gf(products) - Gf(reactants) Gf (kcal/mol) OAA Pyr CO2(g) H+ -190.62 -113.44 -94.45 0(-9.87) 1M pH 7 G = (-113.44 + -94.45) - (-190.62 + 0) = -17.27 kcal/mol In biochemistry-usually deal with physiological conditions (10-7M H+) G ‘ = (-113.44 + -94.45) - (-190.62 + -9.87) = -7.4 kcal/mol Gibb’s Free Energy Since the spontaneity of a reaction is independent of the pathway, we can force G of an overall reaction to be negative even though it has a positive step by coupling it to a step with greater -Gf. Reaction A Method A (steps 6,7) C Pi + NAD+ same as NADH Gº = +1.5 GAP MgADP 1,3-BPG Glyceraldehyde-3phosphate dehydrogenase A B MgATP Gº = -4.5 3PG 3-phosphoglycerate kinase Gº = -3.0 Net reaction GAP 3PG C Gibb’s Free Energy Method B (step 1) C D A B Glucose + Pi MgATP Glucose + Pi + MgATP Net reaction G6P Gº = +3.3 MgADP + Pi Gº = -7.3 G6P + MgADP + Pi Gº = -4.0 • ATP is the energy currency of cells • In phototrophs, light energy is transformed into the light energy of ATP • In heterotrophs, catabolism produces ATP, which drives activities of cells • ATP cycle carries energy from photosynthesis or catabolism to the energy-requiring processes of cells ATP ATP is the general “high energy” coupler molecule used in biochemistry N N O N N O CH2 O O -O-P-O-P-O-P-OO- O- O- MgATP MgADP MgATP AMP HO OH Mg++ Mg Adenosine triphosphate (MgATP) ADP + Pi AMP + Pi AMP + Pi Adenosine + Pi Gº -7.3 -7.4 -7.4 -3.0 ATP O Why is the presence of the energetically unfavorable? O P-O-P anhydride so (1) Ability of products to delocalize electrons (all phosphates) O O P=O -O P O- O -O O- Free ATP (2) Close proximity of charges (bond strain) O O + O + pH 6.0 pH 9.0 Gº 7.89 9.56 0.0 M Mg 0.001 M Mg 0.01 M Mg -8.4 -7.7 -7.5 Adenosine-O-P-O-P-O-P-OO- O- OpK2 ATP-4 Phosphoric Acid Anhydrides • ADP and ATP are examples of phosphoric acid anhydrides • Large negative free energy change on hydrolysis is due to: – electrostatic repulsion – stabilization of products by ionization and resonance – entropy factors Phosphoryl-group Transfer • Energy produced from a rxn can be coupled to another rxn that requires energy to proceed. • Transfer of a phosphate group from high energy phosphorylated compounds can activate a substrate or intermediate of an energy requiring rxn. A-P + ADP -> A + ATP, ATP +C-> ADP + C-P • The ability of a phosphorylated compound to transfer a phosphoryl group is termed its phosphoryl-grouptransfer-potential. Phosphoryl-group Transfer Redox chemistry In addition to energetics -must balance redox chemistry 6 CO2 + 6 H2O Glucose (C6H12O6) + 6 O2 Broken down into “half pathways” Glycolysis Glucose Active hydrogen 2H+ + 2e- 2 pyruvate + 2 (2H) Mitochondria (2H) + 1/2 O2 H2O Common carrier of (H) O N N O N N O HO C-N-H2 O CH2-O-P-O-P-CH2 O O- OOH HO N(+) OH Pi NAD(P) Nicotinamide adenine dinculeotide (phosphate) (oxidized form) NAD+ + 2e- NADH + H+ Common carrier of (H) H H N N O N N O HO C-N-H2 O CH2-O-P-O-P-CH2 O O- OOH O HO N OH Pi NAD(P) Nicotinamide adenine dinculeotide (phosphate) (reduced form) NADH + H+ NAD+ + 2e- Eº ‘ = 0.31 volt Thermodynamically 2e- + 2H+ + 1/2 O2 NADH + H+ H2O NAD+ + 2H+ + 2e- NADH + H+ + 1/2 O2 Eº’ = +0.82 volt Eº’ = +0.31 volt NAD+ + H2O Eº’ = +1.13 volt Convert using the Nernst Equation Ease at which molecule donates electron(s) Gº ‘ = -nF Eº‘ F = faraday= 23,086 cal aka electromotive force n=mol e- Gº ‘ = -2( 23,086 cal mol e- volt mol e- volt )131 volt) Gº ‘ = -56 kcal/mol ATP and NAD(P)H So in metabolism, ATP formed in reaction sequences where Gº‘ > Gº‘ hydrolysis of ATP (catabolism) Used to drive reaction with Gº‘ < Gº‘ hydrolysis (<0) NAD(P)H production and ATP production are usually coupled ATP and NAD(P)H are coenzymes and therefore need to be recycled. Thermodynamics and Metabolism • Standard free energy A + B <-> C + D • Go’ =-RT ln([C][D]/[A][B]) • Go’ = -RT ln Keq • Go’ < 0 (Keq>1.0) Spontaneous forward rxn • Go’ = 0 (Keq=1.0) Equilibrium • Go’ > 0 (Keq <1.0) Rxn requires input of energy