* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download video slide - Northwest Florida State College
Survey
Document related concepts
Butyric acid wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Microbial metabolism wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Transcript
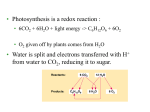
Chapter 9 Cellular Respiration: Harvesting Chemical Energy PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Introduction • Overview: Life Is Work • Living cells – Require transfusions of energy from outside sources to perform their many tasks • Energy – Flows into an ecosystem as sunlight and leaves as heat Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Big Picture • Cells harvest chemical energy stored in organic molecules and use it to generate ATP. • ATP is generated through the transfer of electrons (in the form of H+) from organic molecules. • Two types of Energy Harvesting processes – Aerobic - Cellular Respiration – Anaerobic – Fermentation, Lactic Acid production Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings I. How is ATP produced? • Big Idea: Transfer of electrons releases Energy • Key Question: How do electrons get transferred? A. Redox Reactions: Oxidation and Reduction 1. Definitions a) Oxidation – loss of e from a substance b) Reduction – addition of e to a substance c) Oxidizing Agent – e acceptor d) Reducing Agent – e donor Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings B. Redox reactions in cellular respiration 1. a) Glucose gives up e b) Oxygen accepts electrons 2. e in glucose have a lot of potential E which they give off when transferred to oxygen a) e are transferred in steps, so E is released gradually. 3. Carbs and Fat have a lot of H atoms to free up…therefore they store a lot of E Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings C. Role of NAD+ and Electron Transport Chain 1. NAD+ is the main e transfer molecule in cellular respiration (It is an oxidizing agent since it accepts e) • Dehydrogenases (enzymes) remove a pair of H atoms (2 e) and transfers both e and one proton to NAD+ making NADH • NADH then transfers e to electron transport chain Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 2. Electron Transport Chain (ETC) a) Pathway of several enzymes which pass e from one to another. 1) Everytime e moves from one enzyme to another, energy is released and used to make ATP b) ETC located in the inner membrane of eukaryotes 1) Plasma membrane in prokaryotes c) Oxygen is the final acceptor of e in ETC (making H2O) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 3. Why use the ETC? a) Stepwise release of E is more controlled and better able to be harvested. b) Without ETC, too much E would be released at one time and could not be used as efficiently. H2 + 1/2 O2 Free energy, G Explosive release of heat and light energy Figure 9.5 A Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings H2O (a) Uncontrolled reaction BAD!!!! D. The Stages of Cellular Respiration: A Preview 1. Respiration is a cumulative function of three metabolic stages a) Glycolysis b) The citric acid cycle c) Oxidative phosphorylation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings II. Glycolysis A. Sole purpose is to produce PYRUVATE 1. Occurs in cytosol 2. Breaks down glucose into two molecules of pyruvate (3 C sugars) 3. Two Stages a) Energy investment stage 1) 2 ATP used b) Energy payoff stage 1) 4 ATP produced (Net 2 ATP) 2) 2 NADH produced Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings III. The Citric Acid Cycle • Citric Acid Cycle takes place in the MITOCHONDRION • Prior to entering Citric Acid Cycle, Acetyl CoA is added to the pyruvate molecule Addition of CoA group makes a highly reactive molecule Can give off a lot of Energy (YAY!) CYTOSOL MITOCHONDRION NAD+ NADH + H+ O– S CoA C O 2 C C O O 1 3 CH3 Pyruvate Transport protein Figure 9.10 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings CH3 Acetyle CoA CO2 Coenzyme A Energy Extraction Net Energy Production •8 NADH •2 ATP •2 FADH2 (another e carrier like NAD+) *Note – Carbon skeleton is reused during the next cycle So far only 4 ATP produced total in whole cycle Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings IV. Oxidative Phosphorylation (ETC) A. Electron Transport Chain 1. Thousands of copies in mitochondrion 2. Contains 4 multi-subunit complexes a) As e passed from complex to complex the alternate between reduced and oxidized states b) 10 e carrier molecules in the ETC (most are cytochromes which contain an Fe group that accepts and gives off the e) c) Last cytochrome passes the e to oxygen making H2O Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 3. e are transported into the space between the inner and outer membrane of the mitochondion a) Generates a H+ gradient (high in between membranes, low inside mitocondrion b) When e return to the inside of the mitochondrion, ATP is produced (details soon) 4. Role of NADH and FADH2 a) Donate electrons to the electron transport chain a) FADH2 does not generate as much E because it is a lower energy level carrier b) Eventually powers ATP synthesis via oxidative phosphorylation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings B. Chemiosmosis: The EnergyCoupling Mechanism *oxidative phosphorylation *ATP production (finally) 1. ATP synthase a) Is the enzyme that actually makes ATP b) H+ flows from high concentration to low concentration (passive transport) c) Chemiosmosis – flow of H+ across a membrane Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Electron transport chain and Chemiosmosis Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings C. An Accounting of ATP Production by Cellular Respiration 1. About 40% of the energy in a glucose molecule a) Is transferred to ATP during cellular respiration, making approximately 38 ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings V. Anaerobic Respiration A. Very Similar to Cellular Respiration 1. Have an ETC a) Difference is that O is not the final e acceptor 1) Final acceptors: N, S, and C 2) All of these produce far less E for ATP production. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings VI. Fermentation A. Basic Facts 1. Produces energy without O or ETC. 2. Beginning is the same as cellular respiration a) Glycolysis changes glucose to pyruvate b) Pyruvate is then used to accept e from NADH 1) This free NAD+ so it can go back and produce more ATP in glycolysis. B. Types of Fermentation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Alcoholic Fermentation Pyruvate changed to acetaldehyde. Acetaldehyde accepts 2H and becomes Ethanol. Lactic Acid Fermentation Pyruvate changed directly to lactic acid. In this process, pyruvate accepts 2H. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings C. Fermentation and Cellular Respiration Compared 1. Both use glycolysis to oxidize glucose and other organic fuels to pyruvate a) Generates 2 ATP b) Both use NAD as electron acceptor 2. Recycling NAD is different (how NAD gives up e) a) Cellular Respiration – NAD freed up via ETC (more ATP produced) b) Fermentation – pyruvate eventually accepts e. (No more ATP produced) 3. Result a) cellular respiration - 38 ATP b) Fermentation – 2 ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings • Pyruvate is a key juncture in catabolism Glucose CYTOSOL Pyruvate No O2 present Fermentation O2 present Cellular respiration MITOCHONDRION Ethanol or lactate Figure 9.18 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Acetyl CoA Citric acid cycle VI. Metabolic Connections A. Versatility of Catabolism 1. While glucose is ideal, it can use many other molecules in cellular respiration. a) Proteins, fatty acids, disaccharides, polysaccharides 2. Molecules either converted to glucose or an intermediate form in cellular respiration. a) Amino acids must have amino groups removed via deamination. 3. Beta oxidation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 3. Beta Oxidation a) Breaks fatty acids into glycerol and multiple two carbon molecules b) The 2-C molecules are converted to Acetyl CoA and enter the citric acid cycle. c) Example 18 C fatty acid = 9 acetyl CoA Total of 216 ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings B. Biosynthesis (Anabolic Pathways) 1. The body uses small molecules to build other substances a) Amino acids – proteins b) Carbon fragments for larger molecules 2. These small molecules may come directly from food or through glycolysis or the citric acid cycle (intermediates) a) We can make half of all amino acids from citric acid cycle compounds. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings C. Regulation of Cellular Respiration Cell doesn’t waste energy making stuff if doesn’t need 1. Feedback Inhibition a) End product inhibits an enzyme at the beginning of the process. 1) Excess of ATP slows Cellular Respiration b) Example: phosphofructokinase (allosteric enzyme) 1) Inhibited by ATP and citrate (1st step citric acid cycle) 2) Stimulated by AMP (made from ADP) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings