* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Gene expression wikipedia , lookup
Expression vector wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Magnesium transporter wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Biochemistry wikipedia , lookup
Paracrine signalling wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Protein structure prediction wikipedia , lookup
Interactome wikipedia , lookup
Western blot wikipedia , lookup
Proteolysis wikipedia , lookup
Protein purification wikipedia , lookup
Signal transduction wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Drug design wikipedia , lookup
Protein–protein interaction wikipedia , lookup
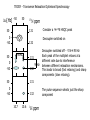
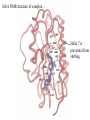
Comparison of T1 and T2 rapid motion (small molecule non-viscous liquids), T1 and T2 are equal Slow motion (large molecules, viscous liquids): T2 is shorter than T1. Problems with higher molecular weights and how to overcome them 1 v T2 v is the linewidth in Hz at half peak height Pg 46 & 47 of Rattle 2H-labeling for molecules greater than 25kDa 1H -reduced relaxation (D/H ~ 1/6.5) Dipole/Dipole relaxation -gives improved signal-to-noise -better resolution 13C H H D H H H N D D D H N TROSY - Transverse Relaxation Optimised Spectroscopy [Hz] -50 50 50 Consider a 1H-15N HSQC peak 131 0 -50 50 ppm Decoupler switched on 132 90Hz 131 0 -50 132 50 131 0 -50 90Hz 132 10.7 10.6 ppm Decoupler switched off - 1J N-H 90 Hz Each peak of the multiplet relaxes at a different rate due to interference between different relaxation mechanisms. This leads to broad (fast relaxing) and sharp components (slow relaxing). The pulse sequence selects just the sharp component The NMR Bandshift and binding site mapping The 1H-15N HSQC spectrum is a very powerful tool for rapid monitoring of binding processes. If the protein is 15N labeled then we monitor chemical shift changes caused by protein-protein interactions, protein DNA interactions, protein-ligand interactions. Examples right. Top, a 1H-15N HSQC of an acyl carrier protein in the apo-form (no fatty acid bound). In the lower panel the effect of increasing fatty acid chain length is monitored. 1. Screen for first ligand 2. Optimise first ligand 3. Screen for second ligand HSQC spectrum of a beta-lactamase in the absence (black) and presence of inhibitor (red) 4. Optimise second ligand 5. Link ligands Schematic of SAR by NMR A case study - Leukocyte function associated protein-1 (LFA-1) This protein is involved in tethering a leukocyte to a endothelium, allowing migration through the tissue to a site of inflammation. One domain of LFA-1, the I-domain is 181 amino acids and undergoes a conformational change where helix 7 slides down the protein, switching it into an active open form. This open form is competent for cell surface binding. If we can stop this switch, we may have an anti-inflammatory mechanism Inflammation (chronic) is responsible for asthma and arthritis. LFA-1 LFA-1 Developed small molecule inhibitors and test binding O- O O N S A B C N N D N O Weak binding mM to mM see a migration of the peaks It is straightforward to derive an expression for F([LTOT]) For the simplest case of a single ligand L, binding to a protein P Kd PL P + L K d dissociation constant for L dissociating from P Define next the average number of ligand molecules bound to each protein, i.e.fraction of protein bound to ligand. conc. of L bound to P/total conc. of P = [PL] [P] + [PL] [P][L] [P] [L] Kd [L] Since [PL] = , then = [P][L] K d [L] Kd [P] + Kd If we measure a chemical shift change going from the free form to the fully bound form then we can know . We also know the amount of ligand we have added, so a suitable plot allows us to determine the K d . Total LFA-1 = 80mM = [P]+[PL] L132 1H shift Total ligand 20 m 50 100 150 200 400 NH of L132 7.487 7.595 7.720 7.796 7.843 7.921 0.087 0.195 0.145 0.325 Bound Ligand Free Ligand 11.6 8.4 26.0 24.0 0.320 0.396 0.443 0.521 100% bound 1H 8.0ppm Unbound 1H 7.4ppm 0.534 0.660 0.738 0.869 42.7 57.3 52.8 59.0 69.5 97.2 141.0 330.5 = [PL] [PL] /80 [P] + [PL] i.e 0.145 * 80 11.6 [PL] 1 Plot 1. vs free ligand concentration 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 Plot 2. Better! 200 300 400 500 [L] (mM) [L] Kd [L] to give the equation of a straight line, 1 [L] K d K d A plot of /[Free Ligand] gives a slope 1 of ,here K d 50mM Kd 0.018 0.016 /[Free Ligand] M- Rearrange = 100 0.014 0.012 0.01 Series1 0.008 0.006 0.004 0.002 0 0 0.2 0.4 0.6 0.8 1 A more successful inhibitor- nM ‘tight’ binding. See unbound and bound populations Solve NMR structure of complex… Helix 7 is prevented from shifting NMR is a diverse tool with which we can study protein structure. It gives us information in solution under ‘physiological’ conditions 2D and 3D techniques combined with modern assignment methods have allowed proteins up to 40 kDa to be solved. The power of NMR lies not just with its ability to solve structures but also its ability to probe binding of ligands and partner proteins in ‘real’ time. Many aspects we have not had time to deal with. NMR reveals how proteins move in solution - can see domains flexing with different timescale motions. These often correlate with binding patches on the protein.