* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Structure and Function of Macromolecules
Basal metabolic rate wikipedia , lookup
Western blot wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Gene expression wikipedia , lookup
Metalloprotein wikipedia , lookup
Point mutation wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Genetic code wikipedia , lookup
Protein structure prediction wikipedia , lookup
Proteolysis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
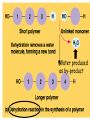
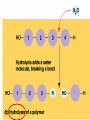
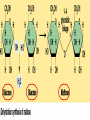
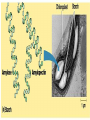
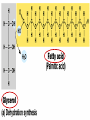
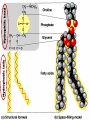
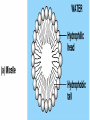
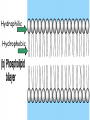
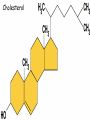
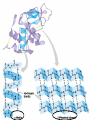
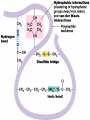
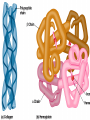
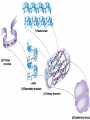
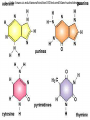
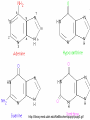
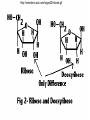
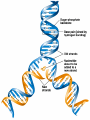
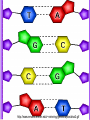
• Macromolecules - larger molecules made from smaller ones. • 4 major classes of macromolecules: carbohydrates, lipids, proteins, and nucleic acids. • 3 of these are polymers because they are made from individual building blocks called monomers. http://www.diabetes.org.nz/pics/carbohydrate_foods.jpg • Monomers - joined together through condensation or dehydration reaction (form macromolecules) • Requires energy; uses covalent bonds (links together monomers) • Water produced. Water produced as by-product • Hydrolysis breaks polymers into monomers. • Water added to polymer; breaks bonds, creates monomers (i.e. digestive process in animals) Carbohydrates • 1Carbohydrates - sugars (monomers) and polymers. • Monosaccharides - simple sugars. • BDisaccharides - double sugars A (monosaccharides linked together) • CPolysaccharides - polymers of monosaccharides. • Sugars named with –ose. • Monosaccharides needed for cellular work. • Help to synthesize other macromolecules. • Maltose - 2 glucose molecules. • Sucrose - 1 glucose, 1 fructose. • Polysaccharides - energy storage. • Starch - energy storage polysaccharide for plants. • Cellulose – polysaccharide; plant cell walls. • Animals store energy as glycogen. • Chitin - polysaccharide - makes up exoskeleton of arthropods (like crustaceans and our sowbugs!). Chitin is used in surgery Lipids • Lipids - no polymers (exception) • Lipids nonpolar (no affinity for water) • Fat made from glycerol and fatty acids. • Saturated fatty acid - No carbon-carbon double bonds in carbohydrate chain. (hydrogen at every possible position) • Form bad fats - solid at room temperature (butter, lard) No double-double bonds • unsaturated fatty acid - 1+ carbon- carbon double bonds. • Formed by removal of hydrogen atoms from carbon skeleton. • Form good fats - liquid at room temperature (oils) • Purpose of fat - energy storage. • Gram of fat stores 2X as much energy as gram of polysaccharide. • Fat also cushions vital organs. • Layer of fat can also function as insulation. http://www.healthline.com/blogs/diet_nutrition/uploaded_images/fat-cat-712938.jpg • MOST IMPORTANT LIPID IN BIOLOGY = Phospholipid • Phospholipids have 2 fatty acids attached to glycerol. • Fatty acid tails are hydrophobic, phosphate group and attachments form hydrophilic head. • When phospholipids added to water, self-assemble with hydrophobic tails pointing toward center, hydrophilic heads on outside. • Phospholipids in cell form bilayer; major component of cell membrane. Hydrophilic Hydrophobic Other Lipids • Steroids - lipids with carbon skeleton consisting of 4 fused carbon rings. • Cholesterol - component in animal cell membranes and hormones. Cholesterol Proteins • Proteins - support, storage, transport, defenses, and enzymes. • Made in ribosomes in cell. • Proteins - amino acids linked together to form polymer. • 20 different amino acids that can be linked together to form thousands of different proteins. http://images.foodnetwork.com/webfood/images/gethealthy/nutritionalallstars/LeanProteins_header.jpg • Amino acids link - polypeptides combine to form proteins. • Amino acids made of hydrogen atom, carboxyl group, amino group, variable R group (or side chain). • R group makes amino acids different from one another. • Shape of protein determines function. • Shapes - 3 dimensional determined by sequence of amino acids. • Primary structure of protein - linear sequence of amino acids determined by genetics. • Secondary structure - two shapes are usually formed: alpha coils or beta sheets. • Tertiary structure determined by interactions among R groups. • Quarternary structure - joining of 2+ polypeptide subunits. • Collagen and hemoglobin examples. • Protein’s shape can change due to environment. • pH, temperature, or salinity (salt concentrations) change - protein can denature (starts to fall apart) • Some proteins can return to functional shape after denaturation, others cannot. Nucleic acids • Amino acid sequence of polypeptide programmed by a gene. • 2 types of nucleic acids: ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). http://www.uic.edu/classes/bios/bios100/lecturesf04am/nucleotides.jpg • DNA gives information so RNA can create proteins. • Flow of genetic information - DNA > RNA -> protein. • Protein synthesis occurs in ribosomes. • Monomers of nucleic acids nucleotides. • Nucleotides made up of 3 parts: nitrogen base, five-carbon sugar, and phosphate group. • Nitrogen bases, rings of carbon and nitrogen, come in 2 types: purines and pyrimidines. • Pyrimidines - cytosine (C), thymine • (T), and uracil (U in RNA only). Purines - adenine (A) and guanine (G). • Pyrimidines - single six-membered ring; purines - five-membered ring. http://library.med.utah.edu/NetBiochem/pupyr/pupy3.gif • In RNA - sugar is ribose; DNA sugar is deoxyribose. • Difference between sugars is lack of oxygen atom in deoxyribose. http://members.aol.com/logan20/ribose.gif • RNA single-stranded - linear shape. • DNA forms double helix. • Sugar and phosphate forms backbone of double helix while nitrogen bases form connection between backbones. • Adenine (A) always pairs with thymine (T) guanine (G) with cytosine (C). • Due to six and five membered rings – shapes are compatible. • Two strands are complementary. http://www.emunix.emich.edu/~rwinning/genetics/pics/dna2.gif • DNA used to show evolutionary similarities between species. • Two species that appear to be closely-related based on fossil and molecular evidence also more similar in DNA and protein sequences than more distantly related species.