* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Bio 110 S.I. chapters 6 & 7
Survey
Document related concepts
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Citric acid cycle wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Transcript
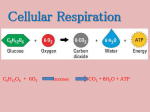
9/21/14 ALL the chemical reactions occurring in the organism including burning food molecules Linking molecules TOGETHER This STORES energy between the bonds BREAKING molecules apart This RELEASES energy 2 things that will determine what happens to a chemical reaction Will the reaction proceed backwards (producing more reactants) or forward (producing products)? IT DEPENDS ON: The amount of energy available This “energy” usually refers to ATP Catalysts (enzymes) can make the reaction proceed faster Ability to promote change Capacity to do work changing one molecule into another molecule Energy kinetic potential chemical associated with physical movement stored in an object due to its position in a: force field system due to its configuration molecular bond 1. First law Law of conservation of energy: Energy cannot be created or destroyed 2. Second law Changing energy from one form to another increases entropy (chaos) Biological processes are not 100% efficient --some energy is converted in heat rather than work! H= Total energy (enthalpy) G + Free energy TS Temperature (K) Unusable energy (entropy) G is negative it is spontaneous This is called an exergonic reaction G is positive it is not spontaneous This is called an endergonic reaction C(s,diamond) ----> C(s,graphite) delta G= - 693 calories C(s,graphite) ----> C(s,diamond) DG= + 693 calories A) C(s,diamond) ----> C(s,graphite) delta G= - 693 calories B) C(s,graphite) ----> C(s,diamond) DG= + 693 calories The Gibb’s Free Energy change in the hydrolysis of ATP is -7.3 kilocalories/mole It is exergonic Spontaneous Favors formation of products The initial amount of energy needed to get the reaction started Activation energy A spontaneous reaction is not necessarily a fast reaction Enzymes are catalysts Catalyst: a name given to something that makes the reaction go faster Catalysts lower activation energy (Ea) Not all catalysts are enzymes- some are ribozymes Usually enzymes are protein Straining bonds in reactants to make it easier to achieve transition state Positioning reactants together to facilitate bonding Changing local environment Direct participation through very temporary bonding 1. Gene regulation 2. Cellular regulation 3. Biochemical regulation 1. Gene regulation Turn on or off genes = turning on and off metabolic pathways 1. Cellular regulation Cell-signaling pathways, like hormones The hormones cause proteins and other molecules in the cell to become active. The result may change the cell permanently or temporarily 3. Biochemical regulation Competitive inhibitors- compete for access to an enzyme’s active site Noncompetitive inhibitors- bind outside the active site When non-competitive inhibitors bind to an allosteric site (somewhere outside active site) it changes the shape of the active site To prevent too much product from being made, non competitive inhibitors stop production before it makes too much Oxidation: Removal of electrons Reduction: Addition of electrons Redox: An electron removed from one molecule is added to another Ae +B→A+ Be Oxidation Is Losing Reduction Is Gaining A is being oxidized It has lost its electron B is being reduced It has gained an electron NOW TO CONFUSE YOU: A is called the REDUCING AGENT B is called the OXIDIZING AGENT Will need to know every detail of Glycolysis pyruvate reduction citric acid cycle electron transport chain fermentation For the test How cells obtain energy from organic molecules (such as sugar) Primary goal is to make ATP and NADH These molecules have high potential energy Organic molecules + O2 → CO2 + H2O + Energy 1. Glycolysis 2. Breakdown of pyruvate to an acetyl group 3. Citric acid cycle 4. Oxidative phosphorylation Can occur with or without oxygen Happens in the cytoplasm Steps are nearly identical in all living species Happens in both eukaryotes and prokaryotes Energy investment 1. Steps 1-3 2 ATP hydrolyzed to create fructose-1,6 bisphosphate Cleavage 2. Steps 4-5 6 carbon molecule broken into two 3 carbon molecules of glyceraldehyde-3-phosphate Energy liberation 3. Steps 6-10 Two glyceraldehyde-3-phosphate molecules broken down into two pyruvate molecules producing 2 NADH and 4 ATP Net yield: 2 ATP Phosphorylated: to add a Phosphate group to a molecule PHASE 1: ENERGY INVESTMENT, Steps 1 - 3 GLUCOSE 1. Glucose is phosphorylated by ATP. This changes glucose to Glucose 6 Phosphate. 2 ATP 2 ADP 2. Glucose 6 Phosphate is rearranged to Fructose 6 phosphate. 3. Fructose 6 Phosphate is FRUCTOSE 1-6 BIPHOSPHATE phosphorylated to make Fructose 1 6 biphosphate PHASE 2: CLEAVAGE, Steps 4 & 5 FRUCTOSE 1 6 BIPHOSPHATE 4. Fructose 1 6 biphosphate is cleaved into 2 molecules: G3P and dihydroxyacetone phosphate Glyceraldehyde 3 Phosphate Dihydroxyacetone phosphate 5. Dihydroxyacetone phosphate is isomerized to G3P Glyceraldehyde 3 Phosphate PHASE 3: ENERGY LIBERATION, Steps 6 - 10 Glyceraldehyde 3 Phosphate 6. G3P is oxidized to make NADH and 1 3 biphosphoglycerate 7. The phosphate is removed from 1 3 biphosphoglycerate to make ATP Pyruvate Glyceraldehyde 3 Phosphate NAD+ NAD+ NADH ADP ATP NADH ADP ATP H20 ADP ATP 8. 3 biphosphoglyerate is rearranged 9. A water is removed H20 ADP ATP 10. A phosphate is removed to make ATP and pyruvate Pyruvate GLUCOSE 2 ATP 2 ADP FRUCTOSE 1-6 BIPHOSPHATE Dihydroxyacetone phosphate G3P NAD+ NADH ADP ATP G3P NAD+ NADH ADP ATP H20 ADP ATP Pyruvate H20 ADP ATP Pyruvate In eukaryotes: pyruvate in transported to the mitochondrial matrix Broken down by pyruvate dehydrogenase Molecule of CO2 removed from each pyruvate Remaining acetyl group attached to CoA to make acetyl CoA 1 NADH is made for each pyruvate CO2 NAD+ NADH Pyruvate Coenzyme A Acetyl CoA