* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Porphyrin Metabolism & Porphyrias
Drug discovery wikipedia , lookup
Lipid signaling wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Butyric acid wikipedia , lookup
Citric acid cycle wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Peptide synthesis wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biosynthesis of doxorubicin wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Amino acid synthesis wikipedia , lookup
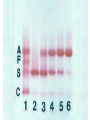
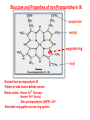
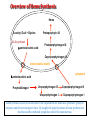
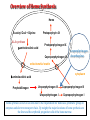
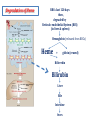
Porphyrin metabolism & porphyrias HMIM224 What are porphyrins ? • • • • • Porphyrins are cyclic compounds that bind metal ions (usually Fe2+ or Fe3+) Porphyrin + Metal = Metaloporphyrin Most prevalent metalloporphyrin in humans is heme (metal here is iron ion) Heme consists of: One ferrous ion (Fe2+) in the centre Protoporphyrin IX (a tetrapyrrole ring) Heme is the prosthetic group of hemoglobin, myoglobin, cytochromes, catalase, tryptophan pyrrolase So, heme + globin protein = hemoglobin Structure of porphyrins • Porphyrins are cyclic molecules formed by 4 pyrrole (tetrapyrrole) rings linked by methenyl bridges. • Different porphyrins vary in the nature of side chains that are attached to each of the 4 pyrrole rings Protoporphyrin IX contains vinyl, methyl & propionate Distribution of side chains - Side chains can be ordered around tetrapyrrole nucleus in 4 different ways designated I, II, III & IV series. Only type III porphyrins are physiologically important in humans - Protoporphyrin IX is a member of type III series • Porphyrinogens are porphyrin precursors intermediate between porphobilinogen & protoporphyrin Porphobilinogen Porphyrinogens Protoporphyrins Methenyl Side bridge Chains Pyrrole ring Structure of Porphyrin Determine the type of porphyrin Structure and Properties of Iron Protoporphyrin IX propionate methyl pyrrole ring vinyl Derived from protoporphyrin IX Pattern of side chains defines isomer Binds metals: Heme- Fe2+ (ferrous) Hemin- Fe3+ (ferric) Zinc protoporphyrin (ZnPP)- Zn2+ Extended conjugation across ring system Biosynthesis of Heme Overview of Heme Synthesis Heme Succinyl CoA + Glycine Protoporphyrin IX ALA synthase -aminolevulinic acid Protoporphyrinogen IX Coproporphyrinogen III mitochondrial matrix cytoplasm -aminolevulinic acid Porphobilinogen Uroporphyrinogen III Uroporphyrinogen I Coproporphyrinogen III Coproporphyrinogen I Heme synthesis occurs in all cells due to the requirement for heme as a prosthetic group on enzymes and electron transport chain. By weight, the major locations of heme synthesis are the liver and the erythroid progenitor cells of the bone marrow. Biosynthesis of heme Site of biosynthesis: Liver & Bone Marrow (erythryoid producing cells) Steps: Step 1: Formation of -amino levulinic acid (ALA): in mitochondria (Rate Controlling Step) Glycine (nonessential amino acid) + Succinyl CoA (intermediate of citric acid cycle) Enzyme: ALA Synthase Coenzyme: Pyridoxal Phosphate (PLP) -Amino levulinic acid (ALA) Biosynthesis of heme (cont.) Clinical importance of first step: When heme (end product) is produced in excessive amounts, heme is converted to hemin. Hemin decreases action of ALA synthase in liver. (end product inhibition). The reverse occurs when heme biosynthesis is reduced. Drugs as grisofulvin (antifungal), hydantoin & phenobarbital (anticonvulsant) increase ALA synthase activity: as these drugs are metabolized by cytochrome p450 in liver resulting in more consumption of heme (component of cytochrome). Accordingly, heme concentration is reduced resulting in stimulation of action of ALA synthase. ALA Synthesis of ALAS 1 Decreased heme Synthesis of CYP 450 is increased Metabolised by CYP 450 Large Amount of Drug intake Biosynthesis of heme (cont.) Cytochrome P450 Monooxygenase System: • Cytochrome P450s (CYPs) are actually a superfamily of related, heme-containing monooxygenase enzymes that participate in abroad variety of reactions. This system performs different functions in two separate locations in cells. • The over-all reaction catalyzed by a cytochrome P450 enzyme is: R-H + O2+ NADPH + H+→R-OH + H2O NADP+ where R may be a steroid, drug, or other chemical. • The name P450 reflects the absorbance at 450 nm by the protein. Role of cytochrome P450 in detoxification of drugs & toxic compounds: • • It may itself activate or inactivate a drug It can make a toxic compound more soluble, thus facilitating its excretion in the urine or feces. Biosynthesis of heme (cont.) Step 2: Formation of porphobilinogen: 2 molecules of -Amino levulinic acid (ALA) condense to form porphobilinogen by the enzyme ALA dehydratase. Clinical importance: ALA dehydratase enzyme is inhibited by heavy metals as lead that results in anemia. (lead poisoning). In this case: ALA in blood is elevated (lab investigation) Biosynthesis of heme (cont.) First & second steps of heme synthesis Biosynthesis of heme (cont.) Further steps: (in mitochondria) Formation of protoporphyrin IX Then, ferrous ions (Fe2+) are introduced into protoporphyrin IX, either: simultaneously or: enhanced by ferrochelatase Clinical importance: Ferrochelatase enzyme is inhibited by lead Biosynthesis of heme (cont.) Further steps of heme synthesis Summary of biosynthesis of heme Glyine Enzyme: + Succinyl CoA STEP 1 ALA Synthase PLP -Amino levulinic acid (ALA) Enzyme: ALA dehydratase. STEP 2 porphobilinogen FURTHER STEPS Protoporphyrin IX Ferrous ion (Fe2+ ) Enzyme: ferrochelatase introduction of iron heme Overview of Heme Synthesis Heme Succinyl CoA + Glycine Protoporphyrin IX ALA synthase -aminolevulinic acid Protoporphyrinogen IX Coproporphyrinogen III uroporphyrinogen decarboxylase mitochondrial matrix cytoplasm -aminolevulinic acid Porphobilinogen Uroporphyrinogen III Uroporphyrinogen I Coproporphyrinogen III Coproporphyrinogen I Heme synthesis occurs in all cells due to the requirement for heme as a prosthetic group on enzymes and electron transport chain. By weight, the major locations of heme synthesis are the liver and the erythroid progenitor cells of the bone marrow. Porphyrias Porphyria are rare inherited defects in heme synthesis. An inherited defect in an enzyme of heme synthesis results in accumulation of one or more of porphyrin precursors depending on location of block of the heme synthesis pathway. These precursors increase in blood & appear in urine of patients. Porphyria means purple colour caused by pigment-like porphyrins in urine of patients. (Diagnosed by lab investigation) Most porphyrias show a prevalent autosomal dominant pattern, except congenital eythropoietic porphyria, which is recessive Porphyrias (cont.) Clinical manifestations of porphyrias: Two types of porphyrias: erythropoietic (bone marrow) & hepatic Hepatic porphyrias are: acute & chronic porrphyrias Generally, individuals with an enzyme defect prior to the synthesis of the tetrapyrroles manifest abdominal and neuropsychiatric signs Those with tetrapyrrole intermediates show photosensitivity with formation of reactive oxygen species (ROS) that damage membranes by oxidation resulting in the following effects: - Skin blisters, itches (pruritis) - Skin may darken, grow hair (hypertrichosis) Porphyria Cutanea Tarda • Chronic hepatic porphyria • The most common type of porphyria • a deficiency in uroporphyrinogen decarboxylase • Clinical expression of the enzyme deficiency is influenced by various factors, such as exposure to sunlight, the presence of hepatitis B or C • Clinical onset is during the fourth or fifth decade of life. • Porphyrin accumulation leads to cutaneous symptoms and urine that is red to brown in natural light and pink to red in fluorescent light Porphyrias (cont.) Acute Hepatic Porphyrias e.g. Acute Intermittent Porphyria • Porphyrias leading to accumulation of ALA and porphobilinogen cause abdominal pain and neuropsychiatric disturbances, ranging from anxiety to delirium. • Symptoms of the acute hepatic porphyrias are often precipitated by administration of drugs such as barbiturates and ethanol. Types of Porphyrias Degradation of Heme Degradation of Heme RBCs last 120 days then, degraded by Reticulo-endothelial System (RES) (in liver & spleen) Hemoglobin (released from RBCs) Heme + globin (reused) Biliverdin Bilirubin Liver Bile Intestine feces Degradation of Heme