* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 10/19
Peptide synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
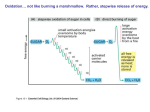
Fermentations NADH must be oxidized to NAD+ in order to oxidize glyceraldehyde-3-P In the absence of an electron transport chain pyruvate or a derivative serves as the electron acceptor for NADH Can lead to the production of some ATP Commonalities of fermentations NADH is oxidized to NAD+ The electron acceptor is often pyruvate or a pyruvate derivative The substrate is partially oxidized Commonalities of fermentations ATP is produced by substratelevel phosphorylation Oxygen is not needed Fermentations Different fermentations are often characteristic of particular microbial groups Alcoholic fermentations Many fungi and some bacteria, algae and protozoa ferment sugars to ethanol and CO2 Pyruvate is decarboxylated to form acetaldehyde Acetaldehyde reduced to form ethanol Lactic acid fermentation Carried out by bacteria, fungi, algae, protozoa and animal muscle cells Pyruvate is reduced to lactate Homolactic fermenters Reduce almost all their pyruvate to lactate using lactate dehydrogenase Heterolactic fermenters Form substantial amounts of products other than lactate Products include lactate, ethanol and CO2 Formic acid fermentation Pyruvate converted to formic acid and other products 2 types of formic acid fermentations: 1. Mixed acid fermentation 2. Butanediol fermentation Mixed acid fermentation Results in the production of ethanol and a mixture of acids including acetic, lactic, succinic and formic acids Formic hydrogenlyase will degrade formic acid to H2 and CO2 Occurs in Escherichia, Salmonella, Proteus and other genera Butanediol fermentation The second type of formic acid fermentation Pyruvate converted to acetoin NADH reduces acetoin to 2,3butanediol Large amounts of ethanol and small amount of mixed acid fermentation acids also produced Butanediol fermentation Characteristic of Enterobacter, Serratia, Erwinia and some species of Bacillus Voges-Proskauer test Differentiates between mixed acid fermenters and butanediol fermenters Detects acetoin Positive for butanediol fermenters and negative for mixed acid fermenters Methyl red test Mixed acid fermenters acidify media to a greater extent than butanediol fermenters Change in color from red to yellow indicates pH has dropped below 4.4 and is read as positive Mixed acid fermenters are positive in the methyl red test Stickland Reaction Some bacteria obtain energy from the fermentation of amino acids One amino acid is oxidized and another is reduced to regenerate NAD+ Acetate, CO2, NH3 and ATP are generated Stickland Reaction Many amino acids can be fermented by this reaction Amino acids can be fermented by other mechanisms besides the Stickland reaction Fermentations Many commercial products are the result of fermentation reactions Alcoholic beverages and bread (alcoholic fermentation) Yogurt, Sauerkraut, Pickles (lactic acid fermentation) Fermentations The tricarboxylic acid cycle Represents stage 3 of catabolism Most of the energy from the complete oxidation of glucose is released in the TCA cycle Also known as citric acid cycle or Kreb’s cycle The tricarboxylic acid cycle Pyruvate is first oxidized, decarboxylated and joined to CoA to form acetyl-CoA Acetyl-CoA combines with oxaloacetate to form citrate The tricarboxylic acid cycle Cycle broken down into three stages: 6 carbon stage 5 carbon stage 4 carbon stage The 6 carbon stage 6 carbon citrate decarboxylated and oxidized to form -ketoglutarate The 5 carbon stage 5 carbon -ketoglutarate is decarboxylated, oxidized and joined to CoA to form succinylCoA The 4 carbon stage 4 carbon succinyl-CoA produces GTP by substrate-level phosphorylation and forms succinate Succinate oxidized by FAD to form fumarate The 4 carbon stage Fumarate malate oxidized by NAD+ to form oxaloacetate Oxaloacetate starts cycle again The tricarboxylic acid cycle Catabolism of carbohydrates, lipids and amino acids results in the production of acetyl-CoA which can be oxidized in the TCA cycle One molecule of acetyl-CoA yields 3 NADH, 1 FADH and GTP 1 The three stages of catabolism (organic molecules) Little ATP synthesized Oxidation of glucose to 6 CO2 4 ATP Most ATP comes from oxidation of NADH and FADH2 in the electron transport chain Fermentation, aerobic and anaerobic respiration Differ regarding the final electron acceptors Electron transport chains Eukaryotic and prokaryotic electron transport chains differ regarding their electron carriers and the details of construction Both operate according to the same basic principles Electron transport chains Electrons move from a carrier with a lower standard reduction potentials (EO) to a carrier with a higher EO Electron transport chains Electrons move from a carrier with a lower standard reduction potentials (EO) to a carrier with a higher EO Mitochondrial electron transport chain Electrons pass from NADH to FMN in complex I Electrons from succinate can be passed to FAD in complex II Both complexes pass electrons to Coenzyme Q (ubiquinone) Mitochondrial electron transport chain Coenzyme Q passes electrons to complex III Electrons passed to cytochrome c then to complex IV Mitochondrial electron transport chain Electrons eventually combine with 1/2 O2 and 2 H+ to form H2O Protons pumped across the membrane at various points during electron transport